Total Phenolic Content and Antioxidant Activities of Leaves and Bark Extract of Adenanthera pavonina L.
Abstract
Adenanthera pavonina L. is an important medicinal plant for Indonesian traditional medicine (“Jamu”). The present study is to determine the phenolic content and evaluate the antioxidant activity of ethyl acetate and ethanolic extracts of leaves and bark of A. pavonina. The total phenolic content was determined using the Folin-Ciocalteu reagent methods, and the antioxidant activity was performed by phosphomolybdate assay, through EC50 values. The highest phenolic content (81.379 mg GAE/g) was found in the hydro-ethanolic extract of the leaves and (80.630 mg GAE/g) in the bark. The antioxidant activity of the two samples was 42.270 μg/mL (leaves) and 45.261 μg/mL (bark). Plants have the potential as drugs with good antioxidant potential and phenolic compound content. Further scientific studies of this plant are supported to evaluate the antioxidant activity using other methods to validate these preliminary experiments properly.
Keywords:
Adenanthera pavonina, Folin-ciocalteu, Phosphomolybdate, SagaIntroduction
Adenanthera pavonina L. is a member of Leguminosae family, subfamily Mimosoideae. The plant is a sizeable perennial tree, a height of 5–10 meters, with many branches, cultivated in some tropical or subtropical countries, including Indonesia. It is called “Saga pohon”; different from “Saga” (Abrus precatorius L.).1 The tree is very famous In India and is known “as redwood” or “red-bead tree”.2 They are generally grown entirely as shade trees or ornamental plants. The seeds are hard coated, red, and attached to the pod to reveal 8–12 inconspicuous and unbroken seeds. The bark is dark brown to greyish, the outside is brown, while the inside is greyish white. The leaves are bipinnate, green on the upper surface and dark green on the underside.3,4 The specific characteristics of the plant parts are essential to authenticate them. The first step must be observed before starting further experiments.5
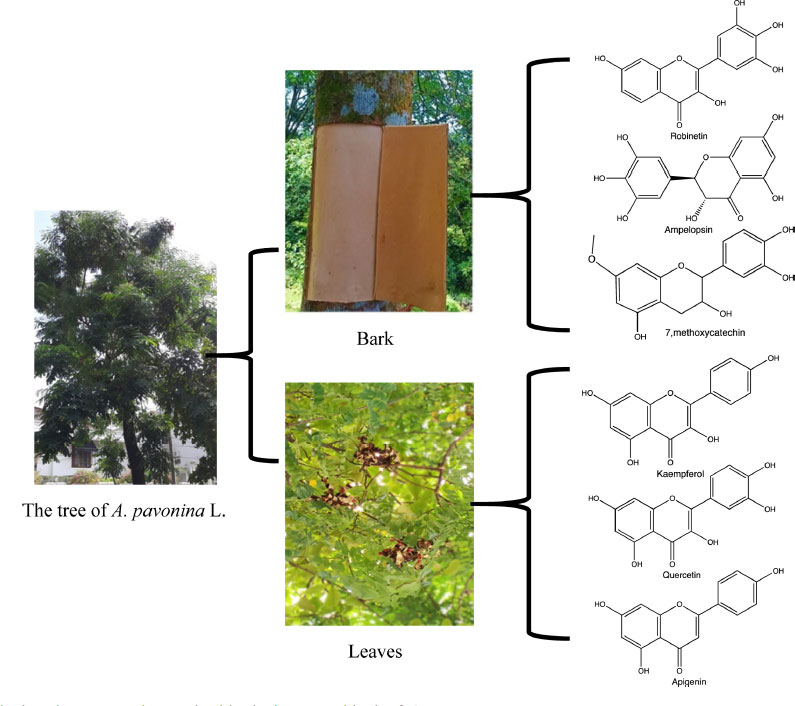
The leaves of A. pavonina L., indicated antibacterial, antifungal, analgesic6 and antioxidant activity,7 and the bark extract has anti-inflammatory activity.8 Numerous global studies are currently focusing on discovering natural antioxidants derived from plants, a polyphenol. Phenolic is an aromatic hydroxylated substance with one or more hydroxyl groups, presenting a sizeable structural diversity divided into different subclasses, such as flavonoids, phenolic acid, tannins, etc.9 Previously reported results of isolation from bark and leaves are shown in (Fig. 1).
Besides the protective effect in biological systems, like anti-bacterial and anti-inflammatory, the phenolic compounds exhibit antioxidant activity.10 Commonly used synthetic antioxidants include butylated hydroxyanisole, butylated hydroxytoluene, and tertiary butylhydroquinone, which are hazardous and carcinogenic.11 Therefore, there is an urgent need for the development and application of more efficient, inexpensive, and naturally derived antioxidants. These natural antioxidants displayed extraordinary biochemical activity and redox potential. The presence of phenolic compounds correlates with a plant's antioxidant action. Among natural antioxidants, phenolics are non-toxic and biodegradable products that have attracted significant attention as the greatest alternative to synthetic antioxidants currently available.12 Earlier scientific investigations of A. pavonina L. have evaluated the antioxidant activity using DPPH method by.13-15
A. pavonina L. has traditionally been used by the people of Indonesia and India for treatment. The previous explanations show that the leaves and bark of this plant have solid chemical properties and pharmacological activity. Scientific determination of leaves and stem bark phenolic content using gradient maceration extraction with hexane, ethyl acetate and ethanol as solvents is unavailable. Previously, many antioxidant tests have been carried out using various methods, but testing using the phosphomolybdate method has never been carried out. This test method determines the antioxidant capacity contained in the sample through the formation of a phosphomolybdenum complex. This research can be a reference for further research on leaves and bark in developing traditional medicine, cosmetics, and food.
Experimental
Chemical and solvents – Hexane, ethanol, ethyl acetate, sodium phosphate, ammonium molybdate, chloroform, methanol, Folin-Ciocalteu’s phenol reagent, and Aluminium Chloride were acquired from Merck (Germany); Quercetin and Gallic acid were obtained from Sigma Aldrich.
Plant material – Fresh leaves and stem bark of A. pavonina L. were collected in June 2021 from the medicinal plant farm of Bogor Agricultural Institute, Bogor. Mr Taopik Ridwan SP, M.Si identified and authenticated the plant in the Tropical Biopharmaca Research Center, Bogor, West Java. A sample frame of the plant has been stored in the laboratory of Pharmacognosy at the Faculty of Pharmaceutical Sciences, the University of Prof. Dr HAMKA, Jakarta.
Extraction and sample preparation – The fresh leaves and stem bark were cleaned with running water to remove dust particles. Samples were dried in the shade at temperatures between 28 and 32°C for ten days before being ground and stored in airtight containers. A portion (1000 g) of the powdered leaves was macerated three times with n-hexane at 24-hour intervals at room temperature and filtered. A rotary evaporator was used to extract and evaporate the solvent. The waste was air-dried and cold macerated with ethyl acetate and 70% ethanol using the same method. The ethyl acetate and 70% ethanol extract were concentrated using a rotary evaporator at reduced pressure and stored at 4°C for further analyses. The yield percentage of hydro-ethanolic and ethyl acetate extract of A. pavonina L. leaves and bark was determined. All the 4 extracts (Ethanolic Extract of A. pavonina L. Leaves = EEL; Ethanolic Extract of A. pavonina L. Bark = EEB; Ethyl Acetate Extract of A. pavonina L. Leaves = EAEL, Ethyl Acetate Extract of A. pavonina L. Bark = EAEB) each were stored in the airtight bottle.
Phytochemical screening – Extracts were checked for compounds using standard phytochemicals (phenols, tannins, flavonoids, alkaloids, terpenes, steroids, and saponins).16
Total water content of extract – The water content was determined using a gravimetric method modified from the Herbal Pharmacopoeia of Indonesia, 2017. The extract of A. pavonina L. leaves and bark was weighed as much as 2–3 g. The extract was dried at 105°C for 5 hours. Drying was carried out to a constant weight. Tests made three replications. The percentage of total water content expressed in % w/w.
Total ash content of extract – The total ash content was determined based on the Herbal Pharmacopoeia of Indonesia, 2017. The extracted sample was 2 g in crucible silica which was heated slowly until the charcoal ran out and turned into ash. Tests made three replications. Ash content expressed in % w/w.17
Total phenolic content – The total phenolic content of all extracts (EEL, EEB, EAEL, and EAEB) was established with the Folin-Ciocalteu reagent in the modified method of Yang et al. (2007), with a gallic acid solution as standard was prepared with variation concentrations: 14, 24, 34, 44, and 54 μg/mL.18 The extract solution was taken with 5 mL of 10% Folin-Ciocalteu reagent and allowed to react for 3 minutes before adding 4 mL of a 7.5% Na2CO3 solution. Before determining the phenol content, the maximum wavelength of gallic acid was measured, and the maximum wavelength was 740.0 nm with an absorbance value of 0.7423 and operating time was carried out to determine the stable measurement time and obtained a stable time at 60 minutes. The absorbance of each mixture was measured at 740 nm after 60 minutes of incubation at room temperature. Standard solutions of gallic acid were likewise subjected to the same technique. Total phenolic content was quantified as mg gallic acid equivalents per g of the extract, using the standard gallic acid curve generated from the leaves and bark of A. pavonina L.
Total antioxidant capacity – Total antioxidant capacity (TAC) was determined as the method proposed by Salamah and Farhana in 201419 with slight modification. The extracts (with various concentrations of 25, 50, 75, 100, and 125 μg/mL) were mixed in a test tube with a 1 mL phosphomolybdate reagent. These test tubes were sealed and incubated at 95°C for 60 min. After allowing the combination to reach room temperature, its absorbance at 695 nm was measured. A Blank containing an equivalent volume of methanol in place of the extracted sample was analyzed using the same procedure. Quercetin is used as a standard and is prepared in various concentrations: 5, 15, 25, 35, and 45 μg/mL. The antioxidant capacity was expressed as mg of quercetin equivalent (QE) per g of extract and EC50 value. Phosphomolybdate reagent (50 mL) contains 3 mL of sulfuric acid, 0.199 g of sodium phosphate, and 0.247 g of ammonium molybdate dissolved in aquadest ad to 50.0 mL.
The absorbance of each sample was inserted into the standard linear regression equation of quercetin. Next, the antioxidant activity of the extract was balanced against the standard quercetin using the following linear or quadratic relationship Y = bx + a. The value of x is entered into the formula below:
| (Eq. 1) |
Where df = dilution factor, v = Volume (mL), m = sample (g).
The antioxidants capacity was computed utilizing the following formula:
| (Eq. 2) |
The EC50 value was calculated using linear regression of the curve between the percent TAC of antioxidant activity and the concentration of the extract of A. pavonina L.
Result and Discussion
The leaves and bark of A. pavonina L. were extracted using a multistage extraction technique using three solvents of varied polarity. The EAEL and EAEB were 9.62% and 10.76%. The EEL and EEB were 21.26% and 25.04%, respectively (Table 1). The polar phytoconstituents dominate in the leaves and bark, which can also mean that the non-phenolic polar compounds are also solved in the polar ethanolic solvent. The maceration extraction method is a traditional method that is still an option for extracting plant phenolic compounds. This method has several advantages because the process is quite simple, the capacity of the extracted sample is larger, and the extraction efficiency can be increased by analyzing time, temperature, and homogenization. The graded maceration extraction method aims to easily separate the chemical components in the sample of A. pavonina L. based on their polarity. In this study, the solvents used were n-hexane, ethyl acetate and ethanol. Using n-hexane removes non-polar compounds, including pigments, waxes, and lipids. Maceration is a common extraction method and can handle various solvents and samples. Despite being a simple classic extraction method, maceration effectively removes phenolic chemicals. Compared to ethyl acetate extract, the yield of ethanolic solvent is greater. Due to the relative polarity of the solvent and the compound being extracted, the ethanolic solvent is superior for extracting substances.
The water and ash content of the ethyl acetate and hydro-phenolic extract of leaves and bark A. pavonina L. is indicated in (Table 1). The water content of extracts was determined using a gravimetric technique. The test determines the extract’s lowest limit (range) water content. The high-water level caused bacteria and fungi to grow and destroy the chemicals in the extract, lowering its quality.20
The water content of the extracted sample is shown as 10% according to Indonesian Herbal Pharmacopoeia guidelines. Ash content testing aims to validate the internal and external mineral content from the process’s beginning to the extract’s formation.21 Organic and inorganic salts are two types of minerals found in a substance. Malic acid, oxalate, acetate, and pectate salts are examples of organic salts. Phosphate, carbonate, chloride, sulfate, and nitrate salts are among the inorganic salts.
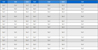
The preliminary phytochemical investigation of the leaves and bark extracts (EEL, EEB, EAEL, and EAEB) revealed the presence of various phytoconstituent (viz. phenols, tannins, flavonoids, alkaloids, terpenes, steroids, and saponins) (Table 2). Polyphenols are frequently extracted from plant matrices with polar solvents. The most acceptable solvents are ethanol, methanol, acetone, and ethyl acetate. Ethanol is a safe and effective solvent for polyphenol extraction. The previous phytochemical screening test was positive for methanolic leaf extracts containing alkaloids, saponins, tannins, steroids,15 phenolics, flavonoids, and terpenoids.22 Previous studies have shown that saga pohon leaves contain polyphenols compound as kaempferol, quercetin, apigenin, flavonol glycoside.23 The bark of A. pavonina L. is known to contain phenolics/flavonoids (i.e. robinetin, ampelopsin, 7-Methoxycatechin),8 alkaloids, saponins, glycosides and phytosterols (i.e. Stigmast-5(6),20(21)-diene-3-one; 6-α-hydroxy stigmast-20(21)-en-3-one; Stigmasta-5,22-dien-3β-ol, stigmasterol glucoside).24
Phytochemical screening of the ethyl acetate and ethanolic extract of leaves and bark of A. pavonina L.
The pharmacological assessment of medicinal plants must include chemical analyses. The presence of secondary metabolites indicates the plant's potential as a pharmaceutical. Many positive pharmacological effects and antioxidant activity are present in the various solvent extracts of the medicinal plant parts.18 Phenolic chemicals from plants, such as flavonoids and tannins, relate to antioxidant activity in biological systems. Due to their redox properties, phenolic compounds are responsible for the antioxidant activity of plant materials, and their hydroxyl groups allow them to function as reducing agents, hydrogen donors, and singlet oxygen quenchers.
The hydro-ethanolic extract of the leaves has a total phenolic content of 81.379 ± 0.53 mg GAE/g when slightly higher than the bark 80.630 ± 1.83 mg GAE/g, while in the ethyl acetate extract were 58.963 ± 0.41 (in leaves) and 63.382 ± 0.20 (in bark). Another study showed that the total phenolic methanol extract of the leaves and bark yielded 8.53 mg/g and 8.51 mg/g,15 leaves 55.43 ± 1.07 μg/mL in GAE.22 Total phenolic content was measured regarding gallic acid equivalent (GAE) in mg per g of extract and expressed as a percentage by weight (% w/w). Plant origin, environmental conditions, plant care operations, processing methods, and analysis procedures can all affect total phenolic levels.
The total phenolic content of A. pavonina L. leaves and barks was evaluated using the Folin-Ciocalteu reagent, which quantifies the amount of TPC present in the amount of phenolic in the sample oxidized by phosphotungstic and phosphomolybdic acids contained in the reagent. Which then produces a blue-coloured solution. The solution's deeper blue hue indicated a higher total phenolic component concentration in the sample. This strategy reduces phosphomolybdate phosphotungstate to a blue tungsten-molybdenum complex by an aromatic core of phenolic chemicals. The total phenolic compounds content is the most preliminary and important step to knowing whether the subject possesses antioxidant properties. The present investigation revealed that EEL and EEB had the highest concentration of phenolic compounds compared to EAEL and EAEB. It is indicated that the polar compounds in the leaf and bark were higher. It can also conduct that the primer metabolite is also solved in the hydro-ethanolic (polar solvent). The hydro-ethanolic solvent has a dielectric constant of 24.30, more significant than the ethyl acetate solvent (6.0), which means the hydro-ethanolic has a more remarkable ability to attract phenolic chemicals from the plant extract. The compatibility of the solvent’s polarity with the chemical being pulled is also significant. Gallic acid is a standard solution because it is a pure phenolic acid with a simple structure and stable characteristics.25 The concentration to create the standard curve was 13–54 μg/mL. The linear equation y = 0.0097x + 0.2274, with the coefficient of determination R2 = 0.9995. Gallic acid’s higher reducing power and weaker chelating ability may contribute to its prooxidant effect.
TAC was determined using phosphomolybdate methods. This assay is based on the reduction of phosphomolybdate ions in the presence of an antioxidant resulting in the formation of a bluish-green phosphate/Molybdenum (V) complex, which is measured spectrophotometrically at 695 nm. For measuring antioxidant activity, the standard comparator is quercetin, which has been widely shown to have antioxidant activity. Extract samples prepared at various concentrations are reacted with phosphomolybdate reagent at 95°C to form a bluish-green complex compound because phenolic ions reduce molybdenum to a molybdenum complex whose absorbance can be read on visible spectrophotometry. Before reading the spectrophotometry, the extracted sample was allowed to stand for the operating time of the extract so that the compounds reacted perfectly with the phosphomolybdate reagent. The molybdenum ion-reducing capacity is based on the spectrophotometric (695 nm) determination of the green phosphate/Mo(V) complex generated because of the antioxidant-mediated reduction of Mo(VI) to Mo(V). The quercetin standard used as a positive control in this antioxidant evaluation was comparatively more effective than the leaves and bark extracts of A. pavonina. The calibration curve of the concentration relationship and absorbance was made using the absorbance measurement of a standard quercetin solution, which showed that the higher the concentration, the greater the absorbance value. The linear regression equation obtained is y = 0.005x + 0.1909, with a correlation coefficient (r) = 0.9997. A value close to 1 indicates a correlation between the concentration of the solution and its absorbance value. Linear regression was then used to calculate the equivalent value of antioxidant activity in A. pavonina L. extract.
EC50 is the effective concentration of the sample capable of reducing radicals by 50%. The EC50 value of the standard quercetin was 19.900 ± 0.029 μg/mL (Fig. 2). These results indicated the best hydro-ethanolic extract of A. pavonina L. leaves (EEL = 42.270 ± 0,532 μg/mL). The ethanolic extract has better radical inhibition properties than the ethyl acetate extract. The equivalence of antioxidant activity was used to determine the strength of the antioxidant activity of leaves and bark extracts against quercetin between concentration and absorbance. These results show that the higher the concentration, the greater the strength of the antioxidant activity against phosphomolybdenum. EEL extract showed the highest total antioxidant capacity of 69.287 mgQE/g, followed by EEB 67.287 mgQE/g, EAEB 45.753 mgQE/g, and EAEL 45.220 mgQE/g.
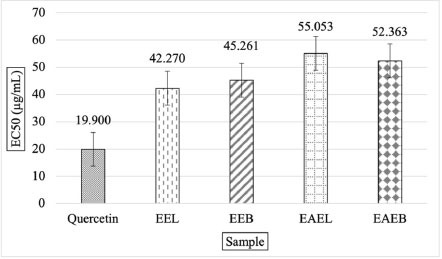
Phosphomolybdenum Assay of the hydro-ethanolic extracts and ethyl acetate extracts of leaves and bark of A. pavonina L.; The picture shows EEL = ethanol extract of leaves; EEL = ethyl acetate extract of leaves; EEB = ethanol extract of bark; EEB = ethyl acetate extract of bark.
Another study carried out that the DPPH scavenging activity of the methanol extract of leaves is 32.31%, which is higher than the bark (30.23%).15 The antioxidant activity (IC50) of dichloromethane, ethyl acetate, and methanol extract of A. pavonina L. was 32.13, 8.72 and 6.44 μg/mL, respectively.13 The aqueous extract of A. pavonina L. has an EC50 value of 7.24 μg/mL, indicating a potent DPPH free radical scavenger.14 In the present results, the leaves and bark ethanolic extract showed better phosphomolybdenum activity than ethyl acetate extract. This result is a straight line with the phenolic content in the ethanolic extract, which is higher than the ethyl acetate extract. Previous research by Mohamed et. al in 2022 showed flavonol glycosides such as isovitixin, quercetin 3-O-(α-ʟ-rhamnopyranosyl-(1→2)-β-ᴅ-xylopyranoside, quercetin 3-O-(α-ʟ-rhamnopyranosyl-(1→2)-β-ᴅ-xylopyranoside showed activity in reducing TNF-α protein levels.26 Several phenolic substances that have been studied display strong antioxidant potential. Additionally, phenolics and flavonoids contribute to the activation of antioxidant enzymes. The ability to increase the production of antioxidant enzymes in the human body induces the antioxidant defense system,27 which is essential in preventing and treating cancer and many other diseases.
The results of this study complete the scientific data regarding the phenolic content and antioxidant activity of the leaves and bark of A. pavonina L. when using the graded extraction method and the antioxidant activity method of phosphomolybdate which have never been tested before. These results indicate that ethanol solvent provides a better ability to withdraw compounds than ethyl acetate, which aligns with the results of its antioxidant activity.
Acknowledgments
The author thanks the Scientific Publication Support and Enhancement Unit (UPPI), University of Muhammadiyah Prof. DR. HAMKA who has helped and provided the opportunity to publish this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- The National Agency of Drug and Food Control (NA-DFC). Monografi Ekstrak Tumbuhan Obat Indonesia Vol 2; BPOM: Indonesia, 2004.
- Mansour, M.; Al-Sayed, E.; El-Gendy, M.; El‐Shazly, M.; Singab, A. N. Arch. Pharm. Sci. A. S. U. 2020, 4, 63–69.
- Mujahid, M.; Ansari, V. A.; Sirbaiya, A. K.; Kumar, R.; Usmani, A. J. Chem. Pharm. Res. 2016, 8, 586–596.
- Geronco, S. M.; Melo, R. C.; Barros, H. L. M.; Aquino, S. R.; de Oliveira, F. C. E.; Islam, M. T.; Pessoa, C. O.; Rizzo, M.; dos Santos Rizzo, M. J. Med. Plants Res. 2020, 14, 24–53.
-
Hanani, E.; Anggia, V.; Amalina, I. N. Pharmacogn. J. 2020, 12, 1317–1324.
[https://doi.org/10.5530/pj.2020.12.181]

- Ahmed, S. M.; Tasleem, F.; Mazhar, F.; Rizvi, S. R. Z. Azhar, I. Pakistan J. Pharmacol. 2018, 35, 57–63.
-
Wickramaratne, M. N.; Punchihewa, J. C.; Wickramaratne, D. B. M. BMC Complement. Altern. Med. 2016, 16, 466.
[https://doi.org/10.1186/s12906-016-1452-y]

-
Saleh-e-In, M. M.; Kar, P.; Ara, A.; Roy, A.; Iriti, M. J. Phytomol. Pharmacol. 2022, 1, 3–18.
[https://doi.org/10.56717/jpp.2022.v01i01.002]

- Hanani E. Pharmacognosy; Penerbit Uhamka Press: Indonesia, 2021, pp 177–178.
- Evans, W. C. Trease and Evans Pharmacognosy 16th Edition; Elsevier: USA, 2009, pp 100, 246, 269–270.
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martinez-Larranaga, M. R.; Wang, X.; Martinez, M.; Anadon, A.; Martinez, M. A. Food Chem. 2021, 353, 129488.
- Babu, D.; Gurumurthy, P.; Borra, S. K.; Cherian, M. J. Med. Plants Res. 2013, 7, 898–905.
- Ara, A.; Saleh-e-In, M. M.; Ahmed, N. U.; Ahmed, M.; Hashem, M. A.; Bachar, S. C. Adv. Nat. Appl. Sci. 2010, 4, 352–360.
-
Silva, I. K.; Soysa, P. Pharmacogn. Mag. 2011, 7, 193–199.
[https://doi.org/10.4103/0973-1296.84229]

- Partha, G.; Rahaman, C. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 30–37.
- Hanani, E. In Analisis Fitokimia(In Bahasa):Phytochemistry Analysis; EGC Medical Publisher: Indonesia, 2015, pp 69, 83, 110, 148–149, 202, 232-233.
- Ministry of Health Republic of Indonesia. Farmakope Herbal Indonesia Edisi II; Ministry of Health Republic of Indonesia: Indonesia, 2017, pp 5–6.
-
Hikmawanti, N. P. E.; Hanani, E.; Sapitri, Y.; Ningrum, W. Pharmacogn. J. 2020, 12, 1311–1316.
[https://doi.org/10.5530/pj.2020.12.180]

-
Salamah, N.; Farahana, L. Pharmaciana 2014, 4, 23–30.
[https://doi.org/10.12928/pharmaciana.v4i1.394]

- Ministry of Health Republic of Indonesia. In Parameter Standar Umum Ekstrak Tumbuhan Obat:Standard Parameter of Medicinal Plant Extract; Departemen Kesehatan Republik Indonesia; Indonesia, 2000, pp 14–17.
-
Yumita, A.; Dwitiyanti.; Ermawati, P. J. Ilm. Farm. Farmasyifa 2022, 5, 41–51.
[https://doi.org/10.29313/jiff.v5i1.8829]

- Mujahid, M.; Siddiqui, H.; Hussain, A.; Rahman, M. D. A.; Khushtar, M.; Jahan, Y. J. Drug Deliv. Ther. 2015, 5, 55–61.
-
Mohammed, R. S.; Abou Zeid, A. H.; El-Kashoury, E. A.; Sleem, A. A.; Waly, D. A. Nat. Prod. Res. 2014, 28, 282–289.
[https://doi.org/10.1080/14786419.2013.856903]

-
Ara, A.; Saleh-E-In, M. M.; Abul Hashem, M.; Ahmad, M.; Hasan, C. M. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 20.
[https://doi.org/10.1186/s43088-019-0013-0]

-
Senet, M. R. M.; Raharja, I. G. M. A. P.; Darma, I. K. T.; Prastakarini, K. T.; Dewi, N. M. A.; Parwata, I. M. O. A. J. Chem. 2018, 12, 13–18.
[https://doi.org/10.24843/JCHEM.2018.v12.i01.p03]

-
Mohamed, S. M.; Hassanein, E. H. M.; Ross, S. A.; Mohamed, N. M. Nat. Prod. Res. 2022, 36, 6267–6278.
[https://doi.org/10.1080/14786419.2022.2027938]

-
Prastiwi, R.; Elya, B.; Hanafi, M.; Sauriasari, R.; Desmiaty, Y.; Dewanti, E.; Herowati, R. Heliyon 2022, 8, e08798.
[https://doi.org/10.1016/j.heliyon.2022.e08798]