Xanthones and 4-Phenylcoumarins from the Twigs of Mesua beccariana (Baill.) Kosterm
Abstract
Two xanthones and 4-phenylcoumarins were isolated from the twigs of Mesua beccariana (Baill.) Kosterm. Among them, one new xanthone, beccarianin A (1), along with 7-isoprenyl-jacareubin (2), mammea A/AA cyclo F (3), and mammea A/BA cyclo F (4). These structures were determined by spectrometric and spectroscopic methods, HRESIMS data, NMR, and UV spectra. Two xanthones (1-2) and two 4-phenylcoumarins (3-4) were evaluated for their cytotoxic effect on the HeLa cells. Compound 1 showed active activity (IC50 = 8.2 μM), and compounds 3-4 showed moderate activity (IC50 = 12.3 and 15.6 μM, respectively).
Keywords:
Mesua beccariana, beccarianin A, xanthone, 4-phenylcoumarin, cytotoxicIntroduction
Mesuabeccariana (Baill.) Kosterm (Calophyllaceae) is a flowering plant found in tropical rainforest Southeast Asia and is commonly called ironwood tree and is used as herbal medicinal. The aqueous decoction of roots or leaves of M. beccariana was used to treat fever, wound medicine, and inflammation.1 The Mesua plants produce diverse phenolic compounds, including chromanone acids, 4-phenyl and 4-propyl coumarins, and xanthone derivatives. The phenolic compounds showed biological activities as an antioxidant, anti-inflammatory, acetylcholinesterase inhibition, and anti-cancer.2-10
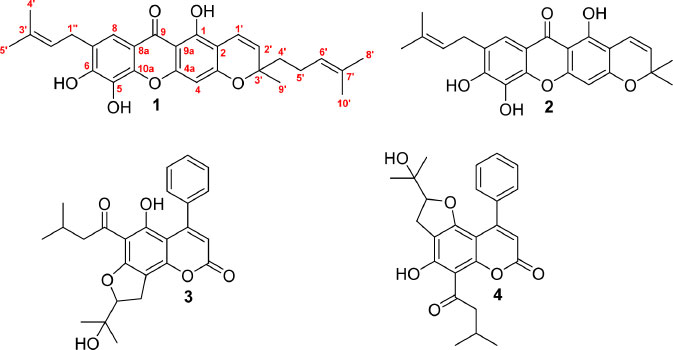
Beccarianin A (1) is a new geranylated xanthone along with 7-isoprenyl-jacareubin (2), mammea A/AA cyclo F (3), and mammea A/BA cyclo F (4) were isolated from M. beccariana twigs (Fig. 1). Two xanthones (1-2) and 4-phenylcoumarins (3-4) from M. beccariana were also reported about the anticancer activity against HeLa cells.
Experimental
General experimental procedures – The wavelength maxima in methanol of compounds 1-4 were measured using a UV spectrophotometer (Shimadzu series 2600i). The chemical formula of the phenolic compounds was recorded using an ESI-TOF mass spectrometer (Waters Corporation - LCT Premier XE). The chemical structure of xanthones was measured by an NMR spectrometer (JEOL ECA-400) operating at 400 MHz (1H NMR spectrum) and 100 MHz (13C NMR spectrum) using TMS as the internal standard and CDCl3 (δH 7.26 and δC 77.1, respectively) as reference standards. Silica gel 60 and PF254 were employed as state phases for gravity column and radial chromatography.
Plant material – The fresh twigs of M. beccariana were collected in Muara Tiga Village, Batu Ampar, Kubu Raya, West Kalimantan, on June 2019. Indonesia. The specimen of plants (MBS 20190617) was identified at the Bogoriense Herbarium, Indonesia, and compared with the same specimen as a reference.
Extraction and isolation – The dried twigs of M. beccariana (0.9 kg) were extracted with 90% methanol for two days (two times) and then partitioned with n-hexane and ethyl acetate. The n-hexane extract (3 g) was separated with column chromatography (CC), using a mixture of n-hexane-ethyl acetate as mobile phase (from 39:1 to 19:1 v/v) to yield compounds 1 (4 mg) and 2 (12 mg). The ethyl acetate extract (10 g) was separated on silica gel by CC, using an n-hexane-acetone gradient (from 19:1 to 4:1 v/v) to yield five fractions A‒E. Compounds 3 (16 mg) and 4 (8 mg) were yielded from fraction C after separation by radial chromatography, eluting with n-hexane-diisopropyl ether gradient (from 9:1 to 1:1 v/v).
Beccarianin A (1) – yellowish solid, = +0.1° (c 0.01, MeOH): UV (MeOH) λmax (log ε) 253 (4.53); 279 (4.47) and 332 (4.12) nm. The NMR data of 1 is shown in Table 1. HRESIMS m/z 463.2126 [M+H]+ (calculated for C28H31O6 for 463.2121).
7-Isoprenyl-jacareubin (2) – yellow solid, UV (MeOH) λmax (log ε) 252 (4.60); 281 (4.61) and 335 nm (4.31). The chemical shift of 3 in the NMR data was compared to the 7-isoprenyl-jacareubin.11
Mammea A/AA cyclo F (3) – yellowish oil, UV (MeOH) λmax (log ε) 234 (4.47); 295 (4.35) and 339 nm (4.30). The NMR data of 3 is very identic to the literature data.12
Mammea A/BA cyclo F (4) – yellowish oil, UV (MeOH) λmax (log ε) 236 (4.42); 298 (4.30) and 338 nm (4.32). The (1H, 13C) NMR spectrum of 4 shows an identical chemical shift to the literature data.8
Cytotoxic assay – The cervical cancer cells (HeLa) were cultivated in RPMI 1640 with 10% fetal bovine serum and 1% penicillin/streptomycin seed in 96-well plates at a density of 5 × 104 cells/cm3. Culture cells were incubated with 5% CO2 at 37°C for 24 hours. All of the isolates (1-4) in well with triplicate of diverse concentrations (100, 50, 10, 5, 1, 0.5, and 0.1 μM) before being incubated for 48 hours at 37°C. After incubation the MTT reagent was added to the culture cells and left on for four hours. The inhibition of cells by the isolates (1-4) was assessed using a microplate reader set to λ 540 nm.13-16 To determine the IC50 values for the isolates (1-4), regression analysis was utilized.
Results and Discussion
Beccarianin A (1) was isolated as a yellowish solid, showing the [M+H]+ ion, with chemical formula C28H31O6, was identified by the HRESIMS at m/z 463.2126 (calcd 463.2121). The UV spectrum of beccarianin A (1) in MeOH (λmax 253, 279, and 332 nm) indicates the chromophore of a xanthone core.17 Two aromatic protons of 1 [δH 6.25 (1H, s, H-4), δC 99.4 (C-4) and 7.60 (1H, s, H-8), δC 117.4 (C-8)], which are indicative of xanthone 1,2,3,5,6,7-hexasubstitued were observed in the NMR data and verified by HMQC and HMBC spectrum. Beccarianin A (1) also showed one hydroxy proton at δH 13.14 (1H, s, 1-OH) and one isoprenyl chain, including a vinyl proton [δH 5.35 (1H, t, J = 7.3 Hz, H-2''), δC 121.0 (C-2'')], a methylene proton [δH 3.43 (2H, d, J = 7.3 Hz, H-1''), δC 28.7 (C-1'')], and two methyl protons [δH 1.78 (3H, s, H-4'', ), δC 25.9 (C-4''), 1.77 (3H, s, H-5''), ), δC 18.0 (C-5'')]. Compound 1, also attributed to the 3'-methyl-5'-isoprenyl-Δ1'-pyran ring consists of three types of vinyl [δH 6.83 (1H, d, J = 10.2 Hz, H-1', δC 115.4 (C-1'), 5.54 (1H, d, J = 10.2 Hz, H-2', δC 126.4 (C-2'), 5.09 (1H, t, J = 7.2 Hz, H-6', δC 123.7 (C-6')], two methylenes [δH 2.09 (2H, m, H-5', δC 22.7 (C-5'), 1.80 (1H, m, H-4'a), 1.69 (1H, m, H-4'b, δC 41.7 (C-4')], and three methyls [δH 1.66 (3H, s, H-8'), δC 25.8 (C-8'), 1.57 (3H, s, H-10'), 1.45 (3H, s, H-9'), δC 27.1 (C-9')]. The 13C NMR spectrum of bec carianin A showed 28 carbon signals that separated, including six oxyaryl carbons [δC 162.8 (C-3), 160.8 (C-1), 158.9 (C-3), 148.4 (C-6), 143.9 (C-10a), 133.9 (C-5)], two methine carbons [δC 117.4 (C-8), 99.4 (C-4)], four carbons [δC 126.4 (C-7), 110.3 (C-8a), 102.5 (C-9a), 101.6 (C-2)], and one carbonyl carbon at δC 180.4 (C-9), indicating that the xanthone 1,2,3,5,6,7-hexasubstitued derivative.
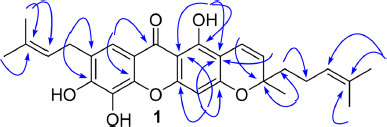
The 3'-methyl-5'-isoprenyl-Δ1'-pyran ring, isoprenyl chain, and hydroxy groups in the xanthone structure were identified by the HMBC spectrum (Fig. 2). An aromatic proton at δH 7.60 (H-8) correlated to a carbon C-7 (δC 126.4), a carbonyl at C-9 (δC 180.4), and an oxyaryl at C-10a (δC 143.9). A methylene proton at δH 3.43 (H-1'') correlated to C-7, an oxyaryl at C-6 (δC 148.4), a carbon at C-3'' (δC 133.7), and two methine carbons at C-8 (δC 117.4) and C-2'' (δC 121.0), supporting the isoprenyl at C-7. An aromatic proton at δH 6.25 (H-5) correlated to two carbons [δC 102.5 (C-9a), 101.6 (C-2)], and two oxyaryls [δC 162.8 (C-3), 158.9 (C-4a)], indicating the 3'-methyl-5'-isoprenyl-Δ1'-pyran ring fused at C-2 and C-3.2 A set vinyl proton supported the 3'-methyl-5'-isoprenyl-Δ1'-pyran linkages in C-2 and C-3 of the xanthone structure. A vinyl proton at δH 6.83 (H-1') correlated to C-1, and an oxycarbon at δC 80.7 (C-3'), a second vinyl proton at δH 5.54 (H-2') corresponded to C-1, and C-3'. The methyl proton at δH 1.45 (H-9') is linked to C-3', a methine carbon at C-2' (δC 126.4), and a methylene carbon at C-4' (δC 41.7). Two methyl protons [δH 1.66 (H-8'), 1.57 (H-10')] correlated to a methine carbon at C-6' (δC 123.7) and carbon signal at C-7' (δC 131.9), supporting the 3'-methyl-5'-isoprenyl-Δ1'-pyran ring in the HMBC spectrum. The structure of 1 was established to be beccarianin A. The name beccarianin A was given based on the name of the plant origin.
The cytotoxic activity of beccarianin A (1), 7-isoprenyl-jacareubin (2), mammea A/AA cyclo F (3), and mammea A/BA cyclo F (4) were assayed against cervical cancer cells (HeLa) by MTT methods. Compounds (1-4) exhibited IC50 values of 8.2 ± 0.2, 60.1 ± 1.2, 12.3 ± 0.4, and 15.6 ± 0.3 μM, respectively. The cells not exposed to the active compound were negative controls, and doxorubicin was a positive control.18-19 The cytotoxic activity suggested that beccarianin A (1) showed active activity, and 7-isoprenyl-jacareubin (2) was inactive. Two 4-phenylcoumarins (3-4) are isomers that showed moderate activity, and compound 3 tends to be more active than 4. The placement of 3-methyl-butanoyl at C-6, furan ring fused at C-7 and C-8 of 3 was slightly more than 3-methyl-butanoyl at C-8, furan ring fused at C-6 and C-7 of 4.
In conclusion, two xanthones (1‒2) and 4-phenylcoumarins (3-4) were isolated from the twigs of M. beccariana. Beccarianin A (1) showed active activity against HeLa cells, and compounds (3-4) were moderate.
Acknowledgments
The research was provided financial assistance by Penelitian Dasar Kompetitif Nasional, No. 922/UN3.15/PT/2022, Universitas Airlangga.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Heyne, K. The useful Indonesian Plants; Research and Development Agency; Ministry of Forestry: Indonesian, 1987, p 2114.
-
Awang, K.; Chan, G.; Litaudon, M.; Ismail, N. H.; Martin, M. T.; Gueritte, F. Bioog. Med. Chem. 2020, 18, 7873–7877.
[https://doi.org/10.1016/j.bmc.2010.09.044]

-
Ee, G. C. L.; Lim, C. K.; Ong, G. P.; Sukari, M. A.; Lee, H. L. J. Asian Nat. Prod. Res. 2006, 8, 567–570.
[https://doi.org/10.1080/10286020500172335]

-
Lim, C. K.; Subramaniam, H.; Say, Y. H.; Jong, V. Y. M.; Khaledi, H.; Chee, C. F. Nat. Prod. Res. 2015, 29, 1970–1977.
[https://doi.org/10.1080/14786419.2015.1015020]

-
Karunakaran, T.; Ee, G. C. L.; Tee, K. H.; Ismail, I. S.; Zamakshshari, N. H.; Peter, W. M. Phytochem. Lett. 2016, 17, 131–134.
[https://doi.org/10.1016/j.phytol.2016.07.026]

-
Rouger, C.; Derbre, S.; Charreau, B.; Pabois, A.; Cauchy, T.; Litaudon, M.; Awang, K.; Richomme, P. J. Nat. Prod. 2015, 78, 2187–2197.
[https://doi.org/10.1021/acs.jnatprod.5b00222]

-
Rouger, C.; Derbre, S.; Richomme, P. Phytochem. Rev. 2019, 18, 317–342.
[https://doi.org/10.1007/s11101-018-9594-9]

-
Tanjung, M.; Rachmadiarti, F.; Saputri, R. D.; Tjahjandarie, T. S. Nat. Prod. Res. 2018, 32, 1062–1067.
[https://doi.org/10.1080/14786419.2017.1378215]

-
Tanjung, M.; Saputri, R .D.; Fitriati, F. F.; Tjahjandarie, T. S. J. Biol. Active Prod. Nature 2016, 6, 95–100.
[https://doi.org/10.1080/22311866.2016.1188726]

-
Teh, S. S.; Ee, G. C. L.; Rahmani, M.; Taufiq-Yap, Y. H.; Go, R.; Mah, S. H. Molecules 2011, 16, 5647–5654.
[https://doi.org/10.3390/molecules16075647]

-
Monache, G. D.; Monache, F. D.; Waterman, P. G.; Crichton, E. G.; de Lima, R. A. Phytochem. 1984, 23, 1757–1759.
[https://doi.org/10.1016/S0031-9422(00)83485-8]

-
Verotta, L.; Lovaglio, E.; Vidari, G.; Finzi, P. V.; Neri, M. G.; Raimondi, A.; Parapini, S.; Taramelli, D.; Riva, A.; Bombardelli, E. Phytochem. 2004, 65, 2867–2879.
[https://doi.org/10.1016/j.phytochem.2004.07.001]

-
Tjahjandarie, T. S.; Tanjung, M.; Saputri, R. D.; Nadar, P. B.; Aldin, M. F.; Permadi, A. Nat. Prod. Sci. 2019, 25, 244–247.
[https://doi.org/10.20307/nps.2019.25.3.244]

-
Saputri, R. D.; Tjahjandarie, T. S.; Tanjung, M. Nat. Prod. Res. 2021, 35, 1256–1261.
[https://doi.org/10.1080/14786419.2019.1644634]

- Tanjung, M.; Tjahjandarie, T. S.; Mardhiyyah, S.; Rahman, G. Z.; Aldin, M. F.; Saputri, R. D.; Ahmat, N. Nat. Prod. Sci. 2022, 28, 58–61.
-
Tanjung, M.; Tjahjandarie, T. S.; Saputri, R. D.; Kurnia, B. D.; Rachman, M. F.; Syah, Y. M. Nat. Prod. Res. 2021, 35, 407–412.
[https://doi.org/10.1080/14786419.2019.1634714]

-
Tanjung, M.; Mujahidin, D.; Hakim, E. H.; Darmawan, A.; Syah, Y. M. Nat. Prod. Commun. 2010, 5, 1209–1211.
[https://doi.org/10.1177/1934578X1000500812]

-
Tjahjandarie, T. S.; Tanjung, M.; Saputri, R. D.; Aldin, M. F.; Susanti, R. A., Pertiwi N. P.; Wibawa, R. S.; Halizah, I. N. Phytochem. Lett. 2021, 44, 78–81.
[https://doi.org/10.1016/j.phytol.2021.06.006]

-
Tanjung, M.; Juliawaty, L. D.; Hakim, E, H.; Syah, Y. M. Fitoterapia 2018, 126, 74–77.
[https://doi.org/10.1016/j.fitote.2017.10.001]