Analysis of the Total Polyphenol, Flavonoid, and Phenolic Acid Contents in Three Different Leaf Types of Lepidium sativum
Abstract
Lepidium sativum (LS) is an annual plant that has been used for the treatment of many ailments. The ethanol extracts of pinnately-lobed (PL), pinnately-compound (PC), and pinnately-veined (PV) leaves of LS were examined for their total polyphenol and flavonoid contents, and their phenolic acid (namely caffeic acid (CA), p-coumaric acid (PA), and ferulic acid (FA)) contents were determined using reverse-phase high-performance liquid chromatography-photodiode array (HPLC/PDA) analysis. Among the three leaf types, the highest average total polyphenol content was found in PV (79.87 mg GAE/g extract), whereas PC showed the highest average total flavonoid content (53.35 mg QE/g extract). According to the HPLC/PDA results, PV exhibited a high amount of CA (78.60 µg/g extract) and FA (1,722.85 µg/g extract), whereas a high content of PA (258.72 µg/g extract) was detected in PC. Higher amounts of the phytochemical compounds PV and PC might be indicative of their superior biological activities compared to PL. To the best of our knowledge, this study is the first to quantify and compare the total polyphenol, flavonoid, and phenolic acid contents in three different leaf types of LS.
Keywords:
Lepidium sativum, total polyphenol, total flavonoid, phenolic acids, HPLC, quantitative analysisIntroduction
Lepidium sativum L. (LS) is a popular edible herbaceous plant that is locally known as garden grass, peppergrass, pepper wart, or watercress.1,2 It is fast-growing and is cultivated worldwide as a food ingredient and medicinal plant.3,4 LS is used abundantly by rural and tribal people for curing many disorders and particularly for preventing postnatal complications.5 It is also used to treat skin diseases, leprosy, hepatopathy, asthma, scurvy, and seminal weakness.6,7 Besides, it has various health-promoting properties, including antidiabetic, antimicrobial, antioxidant, hypoglycemic, anti-inflammatory, antihypertensive, diuretic, antidiarrheal, and anticancer activities, due to the abundance of phytochemicals (e.g. polyphenols, carotenoids, alkaloids, flavonoids, triterpenes, phytosterols, and tocopherols) in it.8-12 Polymorphic LS species have whole or pinnately divided leaves that are differently lobed, frequently with linear segments.13,14 The leaves contain lots of vitamins, proteins, fats, carbohydrates, and minerals and also exhibit anti-inflammatory, antibacterial, and antioxidant activities.15
Polyphenols are a group of naturally occurring chemical compounds found in plants.16 They are characterized by the presence of multiple phenol rings, which are organic chemical structures consisting of a benzene ring with a hydroxyl group.17 There are thousands of different polyphenols, and they have been classified into different subclasses based on their chemical structure.18 Flavonoids, which are a subclass of polyphenols, are the biphenyl propane skeleton of polyphenols. The subgroups of flavonoids are flavones, flavan-3-ols, flavanones, anthocyanidins, and isoflavones.19,20 Another subclass of polyphenols is phenolic acids, which are characterized by the presence of a phenolic ring with a carboxylic acid group.21,22 Polyphenols, flavonoids, and phenolic acids are abundant in various fruits, vegetables, cereals, legumes, nuts, seeds, and beverages such as tea, coffee, and red wine. They have attracted a lot of interest because of their possible health benefits, typically antioxidant properties, anti-inflammatory effects, cardiovascular protection, potential cancer prevention, and positive effects on blood sugar regulation.23-28
Therefore, this study investigated the total polyphenol and flavonoid contents in different leaf types of LS. The samples that showed outstanding results were further analyzed for their phenolic acid contents using high-performance liquid chromatography (HPLC) coupled with a photodiode array (PDA) detector.
Experimental
Plant materials – LS seeds obtained from the National Agrobiodiversity Center, National Institute of Agricultural Sciences, Republic of Korea, were sown and cultivated at EL & I Co., Ltd., Republic of Korea. LS leaves (Fig. 1) were classified into three types based on leaf shapes and margins: pinnately lobed (PL), pinnately compound (PC), and pinnately veined (PV). Among the 151 accessions, the leaf types were classified into 77 accessions of PV, 56 accessions of PL, and 18 accessions of PC. After the quantitative analysis of total polyphenols and flavonoids, a total of 30 accessions were selected for phenolic acid analysis based on the total fresh weight and polyphenol and flavonoid contents of the samples.
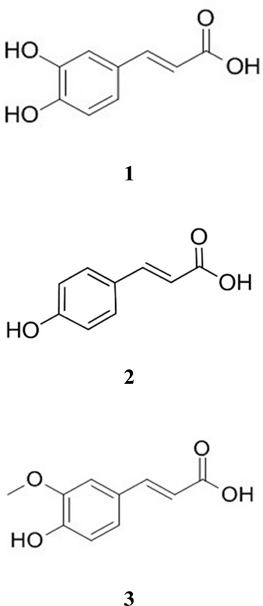
Instruments and reagents – HPLC was performed using an Alliance e2695 Separations Module, Quat with pump, autosampler, and 2998 PDA detector (Waters, Milford, MA, USA). HPLC-grade solvents such as methanol (MeOH), water, trifluoroacetic acid (TFA), and acetonitrile (ACN) were purchased from J. T. Baker (PA, USA). Gallic acid (GA), quercetin, caffeic acid (CA), p-coumaric acid (PA), and ferulic acid (FA) (Fig. 2) were provided by the Natural Product Institute of Science and Technology (www.nist.re.kr), Anseong, Korea.
Sample extraction – Dried LS (3 g) was extracted once for 6 h with ethanol (90 mL) using a reflux extractor. Subsequently, the mixture was filtered and vacuum evaporated to obtain an extract.
Preparation of sample and standard solutions – The extracts of LS and CA, PA, and FA were dissolved in MeOH to obtain a concentration of 50 mg/mL for the extracts and 1 mg/mL for the three standards. Subsequently, they were sonicated for 20 min and filtered using a polyvinylidene fluoride (PVDF; pore size: 0.45 μm) membrane filter. Then, they were diluted using MeOH to obtain concentrations that were appropriate for calculations in the quantitative analysis and were used as standard solutions.
Total polyphenol content – The total polyphenol content of the LS extracts was measured as described in a previous study, with slight modifications.29 Briefly, 60 μL of each sample was mixed with 40 μL of 2 N Folin-Ciocalteu reagent (St. Lewis, Sigma-Aldrich, USA). Next, 100 μL of 7.5% Na2CO3 was added and the samples were allowed to react at room temperature in the dark for 30 min. The absorbance was measured at 760 nm using a microplate reader (Epoch; BioTek, Winooski, VT, USA). The total polyphenol content was calculated based on a standard curve constructed using different concentrations of the standard compound – gallic aicd (GA).
Total flavonoid content – The total flavonoid content of the samples was measured using a modified version of the method described in a previous study.29 Briefly, 100 μL of 2% AlCl3.6H2O was added to 100 μL of the extract and incubated for 10 min. The absorbance was read at 430 nm using a microplate reader (Epoch; BioTek, Winooski, VT, USA). The total flavonoid content was calculated based on a standard curve constructed using different concentrations of the standard compound – quercetin (Q).
HPLC conditions – LS was analyzed quantitatively using a reverse phase HPLC system with an INNO C18 column (25.0 cm × 4.6 mm, 5.0 μm). The column temperature was maintained at 30oC. The mobile phase was composed of 0.1% TFA in water (A) and ACN (B). The elution was performed using a gradient system. The gradient elution conditions were 95% A at 0 min until 8 min, 70% A at 21 min, 30% A at 41 min, 0% A at 47 min, 0% A at 51 min, and 95% A at 55 min until 60 min. The sample injection volume was 10 μL, the mobile phase flow rate was 1.0 mL/min, and the detector wavelength was set at 310 nm.
Calibration curve – As standard solutions, CA, PA, and FA were serially diluted to a minimum of five concentrations to design a calibration curve. The linearity of the calibration curve was determined based on the correlation coefficient (r2), and the content of the target compound was calculated using the equation of the calibration curve. For the calibration correction function for the three compounds, the X-axis (µg/mL) is the concentration, the Y-axis is the peak area, and the value to be substituted is the mean value (n = 3) standard deviation (Table 1).
Results and Discussion
LS is a plant that is familiar to many people since ancient times and is usually consumed as a salad or with other vegetables.30 Several studies have reported that the leaves of this plant have various properties and biological effects that are useful for treating scorbutic diseases, constipation, muscular pain, chest problems, and liver complaints and also as an aphrodisiac.31,32 Moreover, it can combat the human breast cancer cell lines MCF-7 and HEp2. According to the Unani medical system, it can also strengthen the brain and enliven the mind.33 These benefits are due to the presence of secondary metabolites such as sinapic acid, sinapoylglucose, quinic acid, and esters of CA, PA, FA, and flavonoids.34,35
CA, PA, and FA are typical hydroxycinnamic acids, which are one of the main classes of phenolic acids.36 These compounds are widely distributed in various vegetables, beverages, nuts, fruits, herbs, and oils. They are been reported to have many health benefits owing to their antioxidant, anti-inflammatory, anticancer, antiviral, antibacterial, and anti-fibrosis activities.37-39 Furthermore, CA can prevent neurodegenerative diseases and diabetes, and FA can protect against vascular endothelial, kidney, and cardiovascular diseases, inhibit liver cholesterol synthesis, and prevent coronary heart disease and atherosclerosis.40,41 Similarly, PA has analgesic, antiulcer, antiplatelet, and chemoprotective activities.42
A study reported that the polyphenol and flavonoid contents in the MeOH extracts of LS were 993.43 mg GAE/100 g DW and 153.32 mg QE/100 g DW, respectively.43 Another research focused on LS seeds and found a range of total phenolic content from 51.0 to 61.4 mg GAE/100 g fresh weight in different extracts.6 Yet another study examined leaves from various LS populations and found total polyphenol content in the range of 1.25 to 2.36 mg GAE/g extract, along with total flavonoid content ranging from 0.74 to 1.61 mg QE/g extract.44 Additionally, some phenolic and flavonoid compounds were quantified. The results showed that chlorogenic acid was the most abundant compound, followed by FA, CA, kaempferol, and PA. Nevertheless, almost no research has been conducted on the different leaf types of LS.
This study characterized the total polyphenol and flavonoid contents in three different leaf types (PL, PC, and PV) of LS. Overall, the three leaf types showed a high content of both polyphenols and flavonoids. The total polyphenol content ranged from 26.468 to 254.421 mg GAE/g extract, and the highest average concentration was found in PV (79.87 mg GAE/g extract). Meanwhile, the total flavonoid content varied from 25.288 to 87.427 mg QE/g extract, and PC showed the highest average content of flavonoids (53.35 mg QE/g extract).
Based on the results of the total polyphenol and flavonoid contents, 10 samples from each group were selected for phenolic acid (CA, PA, and FA) content evaluation using HPLC/PDA analysis. In the HPLC chromatogram, the retention times of CA, PA, and FA were clearly separated and were at 19.05, 21.80, and 22.81 min, respectively. The results of the HPLC analysis of the three compounds are presented in Fig. 3. The linear calibration curve equations are presented in Table 1. The good linearity of the analytical method was demonstrated by the correlation coefficient (r2), which was more than 0.9997 (Table 1). The contents of the three compounds in all samples were calculated using the calibration curve equations, and the results are presented in Tables 2, 3, and 4. The chromatograms of the samples are illustrated in Fig. 3.
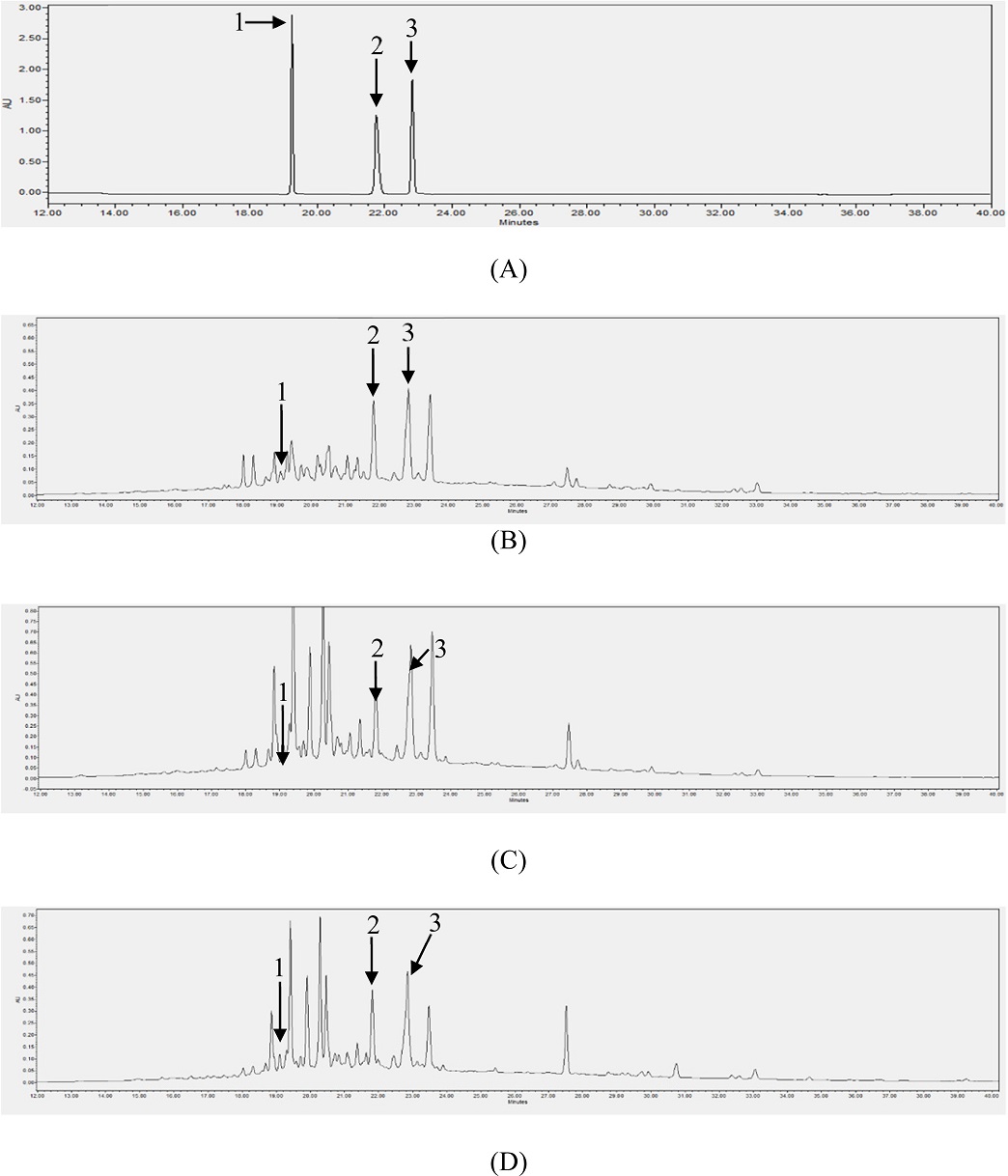
HPLC chromatograms of standards [CA (1), PA (2), and FA (3)] (A), sample PL-44 (B), sample PC-11 (C) and sample PV-42 (D) of LS.
The HPLC analysis showed that the three compounds were detected in all the examined samples. Similar to the results of the total polyphenol and flavonoid content analyses, PC and PV had higher contents of phenolic compounds than PL. Based on the average contents, PV had the highest amounts of CA (78.60 μg/g extract) and FA (1,722.85 μg/g extract), whereas PC had the highest concentration of PA (258.72 μg/g extract). Thus, flavonoid, polyphenol, and phenolic acid contents differ among LS plants with different leaf shapes.
As mentioned above, CA, PA, and FA are compounds with various biological properties; therefore, they provide numerous health benefits and also prevent diseases. In addition to the effects listed above, the consumption of foods rich in CA has also been shown to protect against carcinogenesis (e.g., inhibition of breast cancer cell proliferation).45 By increasing skin elasticity and having a beneficial anti-wrinkle impact, CA may slow down the aging process.46 Furthermore, CA can promote the formation of collagen and prevent premature aging; thus, it is an effective and promising ingredient for the treatment of skin conditions.47 PA is a cosmetic component that can lighten the skin. It has been shown to reduce UV-induced cytotoxicity and hinder the catalytic activity of tyrosinase, particularly human tyrosinase, in vitro.48 Similarly, FA has been proven to combat breast, cervical, lung, pancreatic, colorectal, prostatic, thyroid, bone, and skin cancers.49 Moreover, it can reduce oxidative damage and amyloid pathology, especially in Alzheimer’s disease.50,51 Additionally, as a potential inhibitor of collagen fibrillation and its spread, FA is considered effective against plaques caused by collagen deposition.52
The current study used HPLC/PDA to examine the total polyphenol, flavonoid, and phenolic acid (CA, PA, and FA) contents in three different leaf types (PL, PC, and PV) of LS. Among the three leaf types, the phytochemical content was high in PL but it was not comparable to the phytochemical contents in the other two leaf types; specifically, PV had the highest concentrations of total polyphenol, CA, and FA, whereas PC had the highest content of total flavonoid and PA.
These findings suggest that the three LS leaf types (especially PV) are rich in phytochemicals that exhibit diverse biological activities. Thus, LS leaves are valuable natural materials that can be utilized in the cosmetic and pharmaceutical industries for developing health supplements and anti-aging and anticancerous products.
Acknowledgments
This study was supported by the “Cooperative Research Program for Agriculture Science & Department (Project No. PJ01418503),” Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Kadam, D.; Lele, S. S. Int. J. Biol. Macromol. 2018, 114, 1240-1247.
[https://doi.org/10.1016/j.ijbiomac.2018.04.018]

-
Gill, S. S.; Khan, N. A.; Tuteja, N. Plant Sci. 2012, 182, 112-120.
[https://doi.org/10.1016/j.plantsci.2011.04.018]

- Paranjape, A. N.; Mehta, A. A. Iran. J. Pharmacol. Ther. 2006, 5, 55-59.
-
Najeeb-Ur-Rehman.; Mehmood, M. H.; Alkharfy, K. M.; Gilani, A. H. J. Ethnopharmacol. 2011, 134, 878-883.
[https://doi.org/10.1016/j.jep.2011.01.047]

-
Malar, J.; Chairman, K.; Singh, A. R.; Vanmathi, J. S.; Balasubramanian, A.; Vasanthi, K. Biotechnol. Rep (Amst). 2014, 3, 95-98.
[https://doi.org/10.1016/j.btre.2014.05.006]

-
Aydemir, T.; Becerik, S. J. Food Biochem. 2011, 35, 62-79.
[https://doi.org/10.1111/j.1745-4514.2010.00366.x]

-
Diwakar, B. T.; Dutta, P. K.; Lokesh, B. R.; Naidu, K. A. J. Am. Oil Chem. Soc. 2010, 87, 539-548.
[https://doi.org/10.1007/s11746-009-1523-z]

-
Abdel-Aty, A. M.; Salama, W. H.; Fahmy, A. S.; Mohamed, S. A. Sci. Hortic. 2019, 246, 155-160.
[https://doi.org/10.1016/j.scienta.2018.10.062]

-
Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Food Chem. 2019, 289, 16-25.
[https://doi.org/10.1016/j.foodchem.2019.03.025]

-
Ramadan, M. F.; Oraby, H. F. In Nuts and Seeds in Health and Disease Prevention, Second Edition: Lepidium sativum Seeds: Therapeutic Significance and Health-Promoting Potential; Preedy, V. R.; Watson, R. R. Ed; Academic Press; United Kingdom, 2020, pp 273-289.
[https://doi.org/10.1016/B978-0-12-818553-7.00020-6]

-
Jain, T.; Grover, K.; Kaur, G. Food Chem. 2016, 213, 806-812.
[https://doi.org/10.1016/j.foodchem.2016.07.034]

-
Farhat, M. B.; Amor, G. B.; Beji-Serairi, R.; Selmi, S.; Khammassi, S.; Saidani-Tounsi, M.; Abdelly, C. Int. J. Gastron. Food Sci. 2023, 32, 100736.
[https://doi.org/10.1016/j.ijgfs.2023.100736]

-
Raval, N. D.; Pandya, T. N. Ayu. 2011, 32, 116-119.
[https://doi.org/10.4103/0974-8520.85742]

- Poy, D.; Akbarzadeh, A.; Ghanei, M. J. Hortic. 2015, 103, 130-136.
-
Prajapati, V. D.; Maheriya, P. M.; Jani, G. K.; Patil, P. D.; Patel, B. N. Int. J. Biol. Macromol. 2014, 65, 72-80.
[https://doi.org/10.1016/j.ijbiomac.2014.01.008]

-
Aravind, S. M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Food Res. Int. 2021, 142, 110189.
[https://doi.org/10.1016/j.foodres.2021.110189]

-
Alves-Santos, A. M.; Sugizaki, C. S. A.; Lima, G. C.; Naves, M. M. V. J. Funct. Foods 2020, 74, 104169.
[https://doi.org/10.1016/j.jff.2020.104169]

-
Zhang, H.; Tsao, R. Curr. Opin. Food Sci. 2016, 8, 33-42.
[https://doi.org/10.1016/j.cofs.2016.02.002]

-
Leri, M.; Scuto, M.; Ontario, M. L.; Calabrese, V.; Calabrese, E. J.; Bucciantini, M.; Stefani, M. Int. J. Mol. Sci. 2020, 21, 1250.
[https://doi.org/10.3390/ijms21041250]

-
Briguglio, G.; Costa, C.; Pollicino, M.; Giambo, F.; Catania, S.; Fenga, C. Int. J. Funct. Nutr. 2020, 1, 9.
[https://doi.org/10.3892/ijfn.2020.9]

-
Durazzo, A.; Lucarini, M.; Souto, E. B.; Cicala, C.; Caiazzo, E.; Izzo, A. A.; Novellino, E.; Santini, A. Phytother. Res. 2019, 33, 2221-2243.
[https://doi.org/10.1002/ptr.6419]

-
Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Nutrients 2021, 13, 273.
[https://doi.org/10.3390/nu13010273]

-
Kim, K. H.; Tsao, R.; Yang, R.; Cui, S. W. Food Chem. 2006, 95, 466-473.
[https://doi.org/10.1016/j.foodchem.2005.01.032]

-
Luthria, D. L.; Pastor-Corrales, M. A. J. Food Compos. Anal. 2006, 19, 205-211.
[https://doi.org/10.1016/j.jfca.2005.09.003]

-
Chen, P. X.; Tang, Y.; Marcone, M. F.; Pauls, P. K.; Zhang, B.; Liu, R.; Tsao, R. Food Chem. 2015, 185, 298-308.
[https://doi.org/10.1016/j.foodchem.2015.03.100]

-
Tang, Y.; Zhang, B.; Li, X.; Chen, P. X.; Zhang, H.; Liu, R.; Tsao, R. J. Agric. Food Chem. 2016, 64, 1712-1719.
[https://doi.org/10.1021/acs.jafc.5b05761]

- González-Sarrías, A.; Espín, J. C.; Tomás-Barberán, F. A. Trends Food Sci. 2017, 69, 281-288.
-
Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. J. Food Bioact. 2018, 1, 93-103.
[https://doi.org/10.31665/JFB.2018.1128]

-
Choi, J.; Kim, J.; Lee, H. D.; Cho, H.; Paje, L. A.; Shin, H.; Lee, S. Nat. Prod. Sci. 2022, 28, 27-32.
[https://doi.org/10.20307/nps.2022.28.1.27]

- Singh, B.; Lubhani.; Monzur, M. B.; Sri, M. B.; Arshnoor.; Kaur, J.; Singh, J. J. Pharm. Innov. 2022, 11, 15-25.
-
Al-Snafi, A. E. Int. J. Curr. Pharm. Res. 2019, 11, 1-10.
[https://doi.org/10.22159/ijcpr.2019v11i6.36338]

- Shabbir, F.; Eddouks, M.; Nadeem, F.; Azeem, M. W. Int. J. Chem. Biol. Sci. 2018, 13, 36-45.
-
Ullah, M. A.; Tungmunnithum, D.; Garros, L.; Hano, C.; Abbasi, B. H. J. Photochem. Photobiol. B. 2019, 196, 111505.
[https://doi.org/10.1016/j.jphotobiol.2019.05.002]

- Baregama, C.; Goyal, A. Asian J. Pharm. Clin. Res. 2019, 12, 45-50.
-
Vazifeh, S.; Kananpour, P.; Khalilpour, M.; Eisalou, S. V.; Hamblin, M. R. Biomed. Res. Int. 2022, 2022, 3645038.
[https://doi.org/10.1155/2022/3645038]

-
Kishida, K.; Matsumoto, H. Heliyon. 2019, 5, e02708.
[https://doi.org/10.1016/j.heliyon.2019.e02708]

-
Silva, H.; Lopes, N. M. F. Front. Physiol. 2020, 11, 595516.
[https://doi.org/10.3389/fphys.2020.595516]

-
Stompor-Gorący, M.; Machaczka, M. Int. J. Mol. Sci. 2021, 22, 12889.
[https://doi.org/10.3390/ijms222312889]

-
Yoon, D. S.; Cho, S. Y.; Yoon, H. J.; Kim, S. R.; Jung, U. J. Biomed. Pharmacother. 2021, 142, 111969.
[https://doi.org/10.1016/j.biopha.2021.111969]

-
Alam, M.; Ahmed, S.; Elasbali, A. M.; Adnan, M.; Alam, S.; Hassan, M. I.; Pasupuleti, V. R. Front. Oncol. 2022, 12, 860508.
[https://doi.org/10.3389/fonc.2022.860508]

-
Qi, M. Y.; Wang, X. T.; Xu, H. L.; Yang, Z. L.; Cheng, Y.; Zhou, B. Food Funct. 2020, 11, 3706-3718.
[https://doi.org/10.1039/C9FO02398D]

-
Tehami, W.; Nani, A.; Khan, N. A.; Hichami, A. Dose Response. 2023, 21, 1-9.
[https://doi.org/10.1177/15593258221150704]

-
Mohanad Sultan, N.; Katib, R. Bull. Pharm. Sci. 2021, 44, 377-385.
[https://doi.org/10.21608/bfsa.2021.207156]

- Jelvehgar, N.; Miri, S. M.; Mostafavi, K.; Mohammadi, A. J. Med. Herb. 2023, 14, 37-44.
-
Rosendahl, A. H.; Perks, C. M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J. M. P.; Jernström, H. Clin. Cancer Res. 2015, 21, 1877-1887.
[https://doi.org/10.1158/1078-0432.CCR-14-1748]

- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Bioact. Compd. Health Dis. 2020, 3, 74-81.
-
Magnani, C.; Isaac, V. L. B.; Correa, M. A.; Salgado, H. R. N. Anal. Methods 2014, 6, 3203-3210.
[https://doi.org/10.1039/C3AY41807C]

-
Boo, Y. C. Antioxidants 2019, 8, 275.
[https://doi.org/10.3390/antiox8080275]

- ElKhazendar, M.; Chalak, J.; El-Huneidi, W.; Vinod, A.; Abdel-Rahman, W. M.; Abu-Gharbieh, E. Trop. J. Pharm. Res. 2019, 18, 2571-2576.
-
Fazel Nabavi, S.; Pandima Devi, K.; Sheeja Malar, D.; Sureda, A.; Daglia, M.; Mohammad Nabavi, S. Mini Rev. Med. Chem. 2015, 15, 776-788.
[https://doi.org/10.2174/1389557515666150522102545]

-
Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Nutrients 2015, 7, 5764-5782.
[https://doi.org/10.3390/nu7075246]

-
Jayamani, J.; Naisini, A.; Madhan, B.; Shanmugam, G. Int. J. Biol. Macromol. 2018, 113, 277-284.
[https://doi.org/10.1016/j.ijbiomac.2018.01.225]