Interaction of Icariin from Epimedium koreanum and Udenafil on the Relaxation of Rabbit Penile Corpus Cavernosum Tissue
Abstract
The aim of this study was to investigate the relaxant effect of ethanol extract, fractions from Epimedium koreanum (E. koreanum) on penile corpus cavernosum smooth muscle (PCCSM) in a rabbit. The contractility on PCCSM was also evaluated with icariin, active major compound, from E. koreanum quantified using HPLC analysis. The pre-contracted PCCSM with 10 μM phenylephrine (Phe) was accumulatively treated with extract and fractions (n-hexane, ethyl acetate and n-butanol) from E. koreanum at concentrations of 1, 2, 3 and 4 mg/mL. Icariin was performed with final concentrations of 0.1, 1, 10 and 100 μM. The interaction of icariin with phosphodiesterase-5 (PDE-5) inhibitor, udenafil, was also examined on PCCSM contractility. Extract and three fractions had the relaxant effect on PCCSM in a dose-dependent manner and the maximum relaxation was obtained in ethyl acetate fraction. Icariin dose-dependently relaxed the PCCSM and efficiently enhanced the udenafil-induced relaxation at least two-fold. These results indicate the possibility that icariin from E. koreanum may be a candidate for a new or alternative medicine in erectile dysfunction patients who do not completely respond to PDE-5 inhibitors.
Keywords:
Epimedium koreanum, Icariin, Erectile dysfunction, Phosphodiesterase-5 inhibitor, UdenafilIntroduction
Erectile dysfunction (ED) is defined as the inability of male to achieve and maintain an erection sufficient for satisfactory sexual intercourse.1 The prevalence and degree of ED were correlated with age. While 8% in 40-year-old man has either moderate or absolute ED, this approaches 40% of 60-year-old man.2 Other risk factors associated with ED include diabetes,3–5 cardiovascular disease5,6 and benign prostatic hyperplasia.7
Penile arteries should be dilated and penile corpus cavernosum smooth muscle (PCCSM) should be relaxed for a penile erection, thereby the venous occlusion of penile reduces the blood outflow.8 The parasympathetic nerves release nitric oxide (NO) by the sexual stimulation. NO diffuses into the smooth muscle cells of penile corpus cavernosum arteries and activates a guanylate cyclase. This enzyme changes the guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP).9 cGMP acts on the calcium channels and reduces the intracellular calcium ions level, leading to relaxation of PCCSM.10
Many medical approaches such as hormones, intracavernosal injections, surgery, gene therapy and phosphodiesterase-5 (PDE-5) inhibitors have been used for treating ED.11,12 PDE-5 inhibitors such as tadalafil, sildenafil and udenafil have been used for the first line therapy in ED patients. However, adverse reactions such as headache, visual disorder, facial flushing, dyspepsia and rhinitis have been reported.13 Therefore, it seems worthy to research about the development of new medicines from natural products, that are known to have fewer side effects, with a clinical application in ED.
Epimedium koreanum (E. koreanum), a member of Berberidaceae, has traditionally been used for treating gonadal dysfunction, male impotence and osteoporosis in Korea and China.14–15 Several prenylated flavonol glycosides have been isolated from E. koreanum, including icariin and epimedin A, B, and C.16 Icariin, the principal prenylflavonoid of E. koreanum extract (EKE), has been known as anti-osteoporosis, anti-inflammation and anticardiovascular disease.17,18 Also, it significantly inhibits PDE-5 and induces NO synthase expression in human PCCSM.19,20 Chronic oral treatment with icariin restores the eNOS expression in PCCSM of ED rats and increases the erectile function.21
The purpose of study was to estimate the relaxant effect of ethanol extract from E. koreanum and its active component, icariin, on PCCSM in a rabbit. Additionally, icariin in EKE was quantified using HPLC analysis. The study also examined the relaxant effect of icariin from E. koreanum on PCCSM pre-treated with udenafil.
Experimental
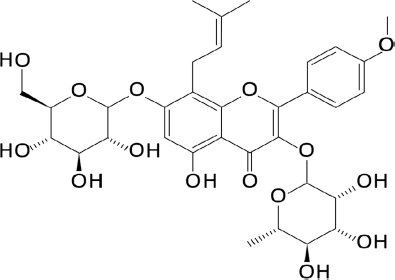
Chemicals and reagents – L-phenylephrine (Phe), dimethyl sulfoxide (DMSO), and HEPES buffer were purchased from Sigma-Aldrich (St. Louis, MO, USA). Udenafil was obtained from Dong-A ST Company (Seoul, Korea). Icariin (Fig. 1), a major compound from E. koreanum, was purchased from ChemFaces (Wuhan, Hubei, PRC). All other reagents and chemicals were purchased from standard suppliers.
Preparation of plant extract and fractions – The dried parts of E. koreanum were purchased from Kyungdong market (Seoul, Korea). The shade-dried E. koreanum (550 g) were grinded to a powder and extracted with 100% ethanol under reflux. After filtration, extract was evaporated in rotary vacuum evaporator (EYELA N-1000, Rikakikai Co., Tokyo, Japan) and lyophilized to yield the total extract (64.1 g). The ethanol extract was partitioned with n-hexane, ethyl acetate and n-butanol. Each of the samples were stored at 4°C until use. EKE and fractions were dissolved in HEPES buffer and diluted to final concentration (1, 2, 3 and 4 mg/mL) in the buffer.
HPLC analysis of icariin in EKE – Icariin standard and EKE were dissolved with 100% methanol. HPLC analysis was carried out on a HPLC analysis system (Hitachi High-Technologies Corporation, Tokyo, Japan) with UV detector at 270 nm. HPLC analysis system was equipped with a Zorbax Eclipse Plus C18 (250 mm × 4.6 mm, 5 μm) analytical column for separation. Acetonitrile was used in mobile phase (A) and distilled water was used in mobile phase (B). HPLC conditions used for the analysis of icariin were shown in Table 1.
Tissue preparation – The male New Zealand white rabbits (2.5-3.0 kg) were individually housed in an optimized environment at 18± 1°C under relative humidity of 62 ± 5%. These animals were acclimated for the first week and randomly assigned into the group, respectively. The rabbits were intravenously anesthetized with 25 mg/kg xylazine hydrochloride and 50 mg/kg ketamine and they were exsanguinated. The penis was removed rapidly and PCCSM was carefully anatomized from the surrounding tunica albuginea. During the procedure, each step was undertaken prudently to prevent the overstretching of tissue or the damage to functional endothelium. All procedures for animals was performed in accordance with the rules of use and care of laboratory animals guide approved by the Institutional Animal Care and Use Committee of Jeonbuk National University Laboratory Animal Center (cuh-IACUC-2016-12).
Organ chamber studies – PCCSM strip of 1.5 × 1.5 × 7 mm was vertically placed in a 2 mL organ chamber. One end was secured with a thread to the tip of a force transducer (FT03, Grass Telefactor; West Warwick, RI, USA), and the other end was connected for isometric tension measurement with a thread to a holder. The organ chamber containing a HEPES buffer was continuously aerated with 100% O2 at 36°C. The strip was equilibrated with several modifications of length for 60 min until a baseline force stabilized at 1 g, and the oxygenated solution was replaced every 15 min. After stabilization, 10 μM Phe was added to control the maximal contractile tension, and the samples with desired final concentration were added to the organ chamber. The relaxant effects of extract and fractions from E. koreanum at concentrations of 1, 2, 3 and 4 mg/mL were measured by cumulative addition from the plateau of Phe-induced contraction. Icariin was measured with final concentrations of 0.1, 1, 10 and 100 μM. The variation in isometric force was executed and recorded using the PowerLab data 400 acquisition system (Software Chart, version 5.2; AD Instruments, Castle Hill, Australia).
Interaction of icariin with udenafil on PCCSM tension – PCCSM tissue was pre-treated with PDE-5 inhibitor, 0.1 μM udenafil, for 30 min and then 0.1 μM icariin was added to the organ chamber after Phe-induced contraction. Contrarily, PCCSM was pre-incubated with icariin for 30 min and then udenafil was added to the organ chamber of tissue after Phe-induced contraction.
Statistical analysis – The Phe-induced penile contractile responses were considered to be the 100% values and all following responses to EKE, fractions or icariin were expressed as a percentage of value. All results were presented as the means ± standard derivations (SD) and n refers to the number of tissues. The statistical significance was analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. A value of p < 0.05 was considered to be statistically significant.
Results and Discussion
To investigate the responses of cumulative relaxation to ethanol extract and fractions from E. koreanum, tension measurement was performed on pre-contracted PCCSM with Phe. As shown in Fig. 2, the ethanol extract from E. koreanum had concentration-dependently the relaxant effect and the maximum relaxant effect was obtained at 4 mg/mL. The relaxations induced through 1, 2, 3 and 4 mg/mL of EKE were 30.65 ± 2.10, 44.74 ± 3.72, 58.88 ± 4.43 (p < 0.05) and 75.23 ± 3.65% (p < 0.01), respectively. The relaxant effects of n-hexane, ethyl acetate and n-butanol fractions from E. koreanum were measured on Phe pre-contracted PCCSM. All three fractions significantly relaxed PCCSM in a concentration-dependent manner, with a maximum relaxant effect observed at 4 mg/mL. Among the three fractions, the ethyl acetate fraction had the most significant relaxant effect. The relaxation induced by 1-4 mg/mL of ethyl acetate fraction was 40.48± 2.48%, 61.22 ± 5.43% (p < 0.01), 76.09 ± 2.31% (p < 0.01) and 85.82 ± 4.97% (p < 0.01), respectively (Fig. 3).
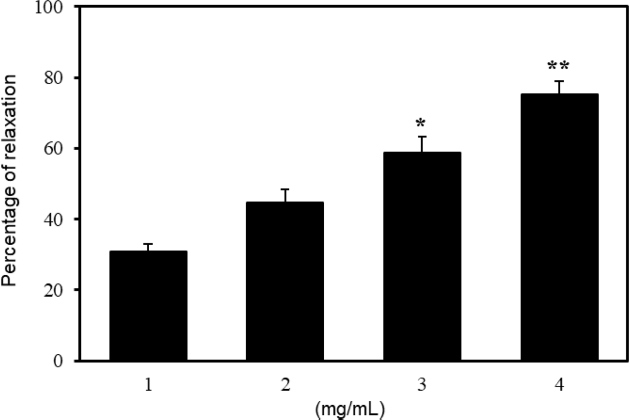
Percentage of relaxation induced by Epimedium koreanum extract (EKE) (n = 6). The penile corpus cavernosum smooth muscle (PCCSM) contracted with 10μM L-phenylephrine (Phe) was treated at four concentrations of EKE (1, 2, 3 and 4 mg/mL). Statistical analysis was carried out by ANOVA followed by Bonferroni’s test (*p < 0.05 vs 1 mg/mL, **p < 0.01 vs 1 mg/mL).
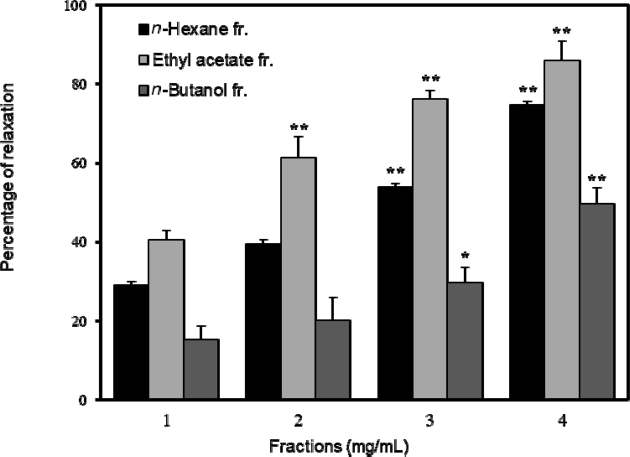
Percentage of relaxation induced by n-hexane, ethyl acetate and n–butanol fractions from Epimedium koreanum extract (EKE) (n = 6). The penile corpus cavernosum smooth muscle (PCCSM) contracted with 10μM L-phenylephrine (Phe) was treated at four concentrations of fractions from EKE (1, 2, 3 and 4 mg/mL). Statistical analysis was carried out by ANOVA followed by Bonferroni’s test (*p < 0.05 vs 1 mg/mL, **p < 0 .0 1 vs 1 mg/mL).
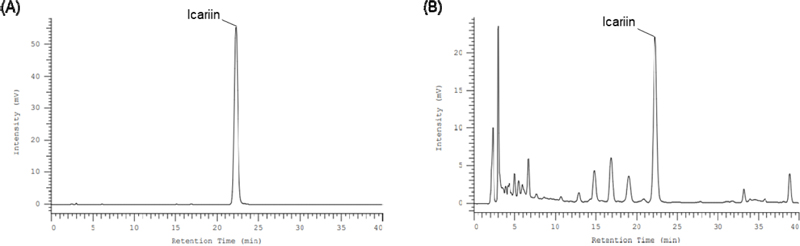
To identify the active component responsible for the relaxation effect of extracts and fractions of E. koreanum, we examined the content of icariin, which is abundantly present in the ethyl acetate fraction known for its excellent relaxant effect22, and its impact on PCCSM relaxation. As shown in Fig. 4A, the peak of icariin standard was appeared at 22 min and detected well. The peak of icariin compared to that of EKE in a chromatogram was appeared at same retention time and separated well without interruption by other peaks (Fig. 4B). The calibration curves of standards were calculated as regression equations (y = ax+b). The correlation coefficient (R2) of icariin was 0.999 and it demonstrated the calibration curve of icariin standard is linear (Table 2). In addition, the accuracy and precision of the method were determined by measuring intra-day and inter-day variations. The values of accuracy and precision were within acceptable ranges. Intra-day data was evaluated by conducting three injections of icariin at three different concentrations on the same day, respectively. The procedures used for intra-day were repeated and performed for three consecutive days to verify the inter-day data. The variations were expressed as the relative standard deviation (RSD), and the summarized results are shown in Table 3. To calculate concentration of icariin in EKE, the linear calibration curve of icariin standard was used. The content of icariin in EKE was observed 4.16 ± 0.18% (Table 2).
The relaxant effect of icariin, active compound of E. koreanum, was performed to investigate the cumulative relaxation on the Phe pre-contracted PCCSM. Icariin with a range of 0.1, 1, 10 and 100 μM relaxed dose-dependently the PCCSM with 24.30 ± 2.71, 52.93 ± 3.16 (p < 0.01), 69.70 ± 3.76 (p < 0.01) and 91.59 ± 2.19% (p < 0.01), respectively (Fig. 5). It was confirmed that icariin was contained in extract and fractions of E. koreanum, with the highest amount being detected in the ethyl acetate fraction, which had the most relaxing effect.23 Therefore, it was shown that icariin is an active component of E. koreanum which has PCCSM relaxation effect.
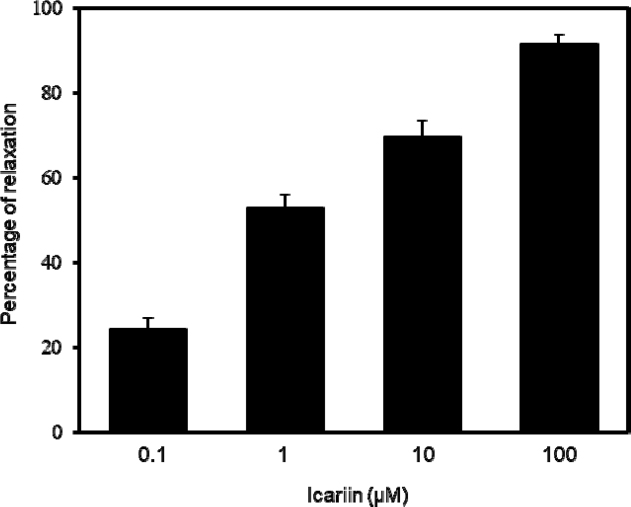
Percentage of relaxation induced by icariin (n = 6). The penile corpus cavernosum smooth muscle (PCCSM) contracted with 10μM L-phenylephrine (Phe) was treated at four concentrations of icariin (0.1, 1, 10 and 100 μM). Statistical analysis was carried out by ANOVA followed by Bonferroni’s test (**p < 0 .0 1 vs 0.1 μM of icariin).
The relaxation induced by a single dose of 0.1 μM udenafil on Phe pre-contracted PCCSM was 11.41 ± 1.53% and the single use of 0.1 μM icariin-induced relaxation on Phe pre-contracted PCCSM was 10.51 ± 2.51% (Fig. 6). The relaxation of icariin was 22.09 ± 1.27% (p < 0.01) on Phe pre-contracted PCCSM, that had been pre-incubated with udenafil. The relaxation of udenafil was 20.77 ± 1.68% (p < 0.05) on Phe pre-contracted PCCSM, that had been pre-incubated with icariin (Fig. 6). Icariin efficiently enhanced the udenafil-induced relaxation at least two-fold. Our results showed that the relaxation effect of 0.1 μM icariin and 1 μM icariin was increased by two times. It was confirmed that the relaxation effect of icariin alone and the relaxation effect of icariin in combination with udenafil were doubled by the synergistic action between the components, not additive action by the dose.
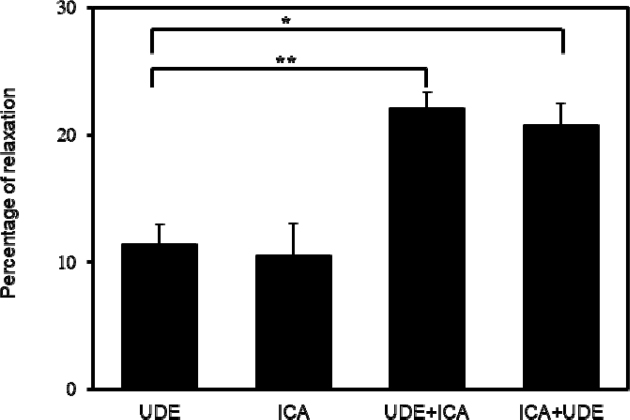
Interaction of icariin (ICA, 0.1 μM) with udenafil (UDE, 0.1 μM) (n = 6). UDE + ICA means the relaxation induced by icariin in pre-contracted penile corpus cavernosum smooth muscle (PCCSM) with udenafil. ICA + UDE means the relaxation induced by udenafil in pre-contracted PCCSM with icariin. Statistical analysis was carried out by ANOVA followed by Bonferroni’s test (*p < 0.05 vs UDE, **p < 0.01 vs UDE).
Although many medicines and therapies have been used for treating ED, discovering a new medicine or an alternative medicine is still an important study. An oral PDE-5 inhibitor including udenafil has been commonly used for treating ED patients.23,24 Although oral PDE-5 inhibitors are effective to a wide range of ED patients, they are not efficacious in all patients.25 It has been reported that the treatment with PDE-5 inhibitors improved approximately 30% to 50% ED patients.26 Many researchers are also searching for new or alternative medicines to improve erectile function.
Our present study showed that the ethanol extract and fractions from E. koreanum dose-dependently relaxed the Phe pre-contracted PCCSM. The relaxation induced by icariin, which was the major compound in E. koreanum, was in a dose-dependent manner. Furthermore, icariin significantly enhanced the udenafil-induced relaxation on PCCSM. Conclusively, these results present the possibility that icariin from E. koreanum may be a promising candidate for a new or alternative medicine in ED patients who do not totally respond to PDE-5 inhibitors. Additionally, the quantitative analysis of icariin using HPLC method could be applied to the fundamental data for the standardization of products from E. koreanum.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NO. 2021R1F1A1045720).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
NIH Consensus Conference. JAMA. 1993, 270, 83−90.
[https://doi.org/10.1001/jama.270.1.83]

-
McKinlay, J. B. Int. J. Impot. Res. 2000, 12, S6−S11.
[https://doi.org/10.1038/sj.ijir.3900567]

-
Bacon, C. G.; Hu, F. B.; Giovannucci, E.; Glasser, D. B.; Mittleman, M. A.; Rimm, E. B. Diabetes Care 2002, 25, 1458−1463.
[https://doi.org/10.2337/diacare.25.8.1458]

-
De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, Pellegrini F, Sacco M, Tognoni G, Valentini M, Nicolucci A. Diabetes Care 2002, 25, 284−291.
[https://doi.org/10.2337/diacare.25.2.284]

-
Ledda, A. Curr. Med. Res. Opin. 2000, 16, S17−S20.
[https://doi.org/10.1185/0300799009117035]

-
Burchardt, M.; Burchardt, T.; Anastasiadis, A. G.; Kiss, A. J.; Shabsigh, A.; de La Taille, A.; Pawar, R. V.; Baer, L.; Shabsigh, R. Int. J. Impot. Res. 2001, 13, 276−281.
[https://doi.org/10.1038/sj.ijir.3900725]

-
Braun, M. H.; Sommer, F.; Haupt, G.; Mathers, M. J.; Reifenrath, B.; Engelmann, U. H. Eur. Urol. 2003, 44, 588−594.
[https://doi.org/10.1016/S0302-2838(03)00358-0]

-
Fazio, L.; Brock, G. Can. Med. Assoc. J. 2004, 170, 1429−1437.
[https://doi.org/10.1503/cmaj.1020049]

-
Andersson, K. E.; Wagner, G. Physiol. Rev. 1995, 75, 191−236.
[https://doi.org/10.1152/physrev.1995.75.1.191]

-
Kim, N.; Azadzoi, K. M.; Goldstein, I.; Saenz de Tejada, I. J. Clin. Invest. 1991, 88, 112−118.
[https://doi.org/10.1172/JCI115266]

-
Mobley, D. F.; Khera, M.; Baum, N. Postgrad. Med. J. 2017, 93, 679−685.
[https://doi.org/10.1136/postgradmedj-2016-134073]

-
Yafi, F. A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A. M.; Goldfarb, S.; Maggi, M.; Nelson, C. J.; Parish, S.; Salonia, A.; Tan, R.; Mulhall, J. P.; Hellstrom, W. J. Nat. Rev. Dis. Primers 2016, 2, 16003.
[https://doi.org/10.1038/nrdp.2016.3]

-
Stroberg, P.; Murphy, A.; Costigan, T. Clin. Ther. 2003, 25, 2724−2737.
[https://doi.org/10.1016/S0149-2918(03)80329-6]

-
Kang, H. K.; Choi, Y. H.; Kwon, H.; Lee, S.-B.; Kim, D.-H.; Sung, C. K.; Park, Y. I.; Dong, M. S. Food Chem. Toxicol. 2012, 50, 2751−2759.
[https://doi.org/10.1016/j.fct.2012.05.017]

-
Makarova, M. N.; Pozharitskaya, O. N.; Shikov, A. N.; Tesakova, S. V.; Makarov, V. G.; Tikhonov, V. P. J. Ethnopharmacol. 2007, 114, 412−416.
[https://doi.org/10.1016/j.jep.2007.08.021]

-
Su, X. D.; Li, W.; Ma, J. Y.; Kim, Y. H. Nat. Prod. Res. 2018, 32, 2347−2351.
[https://doi.org/10.1080/14786419.2017.1405412]

-
He, C.; Wang, Z.; Shi, J. Adv. Pharmacol. 2020, 87, 179−203.
[https://doi.org/10.1016/bs.apha.2019.10.004]

-
Meng, F.-H.; Li, Y.-B.; Xiong, Z.-L.; Jiang, Z.-M.; Li, F.-M. Phytomedicine 2005, 12, 189−193.
[https://doi.org/10.1016/j.phymed.2004.03.007]

-
Ning, H.; Xin, Z.-C.; Lin, G.; Banie, L.; Lue, T. F.; Lin, C.-S. Urology 2006, 68, 1350−1354.
[https://doi.org/10.1016/j.urology.2006.09.031]

-
Jin, F.; Gong, Q.-H.; Xu, Y.-S.; Wang, L.-N.; Jin, H.; Li, F.; Li, L.-S.; Ma, Y.-M.; Shi, J.-S. Int. J. Neuropsychopharmacol. 2014, 17, 871−881.
[https://doi.org/10.1017/S1461145713001533]

- Tian, L.; Xin, Z. C.; Liu, W. J.; Yang, Y. M.; Liu, G.; Chen, L.; Fu, J.; Wang, L. L. Natl. Med. J. China 2004, 84, 954−957.
-
Zhang, W.; Chen, H.; Wang, Z.; Lan, G.; Zhang, L. J. Food Sci. Technol. 2013, 50, 1122−1129.
[https://doi.org/10.1007/s13197-011-0447-4]

- Salem, E. A.; Kendirci, M.; Hellstrom, W. J. Curr. Opin. Investig. Drugs 2006, 7, 661−669.
-
Yuan, J.; Zhang, R.; Yang, Z.; Lee, J.; Liu, Y.; Tian, J.; Qin, X.; Ren, Z.; Ding, H.; Chen, Q.; Mao, C.; Tang, J. Eur. Urol. 2013, 63, 902−912.
[https://doi.org/10.1016/j.eururo.2013.01.012]

-
Deng, W.; Bivalacqua, T. J.; Hellstrom, W. J.; Kadowitz, P. J. Int. J. Impot. Res. 2005, 17, S57−S63.
[https://doi.org/10.1038/sj.ijir.3901430]

-
Hatzichristou, D.; Rosen, R. C.; Broderick, G.; Clayton, A.; Cuzin, B.; Derogatis, L.; Litwin, M.; Meuleman, E.; O'Leary, M.; Quirk, F.; Sadovsky, R.; Seftel, A. J. Sex. Med. 2004, 1, 49−57.
[https://doi.org/10.1111/j.1743-6109.2004.10108.x]