In-silico Studies of Boerhavia diffusa (Purnarnava) Phytoconstituents as ACE II Inhibitor: Strategies to Combat COVID-19 and Associated Diseases
Abstract
COVID-19 caused a catastrophe in human health. People infected with COVID-19 also suffer from various clinical illnesses during and after the infection. The Boerhavia diffusa plant is well known for its antihypertensive activity. ACE-II inhibitors and calcium channel blockers are reported as mechanisms for the antihypertensive activity of B. diffusa phytoconstituents. Various studies have said ACE-II is the virus's binding site to attack host cells. COVID-19 treatment commonly employs a variety of synthetic antiviral and steroidal drugs. As a result, other clinical illnesses, such as hypertension and hyperglycemia, emerge as serious complications. Safe and effective drug delivery is a prime objective of the drug development process. COVID-19 is treated with various herbal treatments; however, they are not widely used due to their low potency. Many herbal plants and formulations are used to treat COVID-19 infection, in which B. diffusa is the most widely used plant. The current study relies on discovering active phytoconstituents with ACE-II inhibitory activity in the B. diffusa plant. As a result, it can be used as a treatment option for patients with COVID-19 and related diseases. Different phytoconstituents of the B. diffusa plant were selected from the reported literature. The activity of phytoconstituents against ACE-II proteins has been studied. Molecular docking and ligand-protein interaction computation tools are used in the In-silico experiment. Physicochemical, drug-likeness, water solubility, lipophilicity, and pharmacokinetic parameters are used to evaluate phytoconstituents. Liriodenine has the best drug-likeness, bioactivity, and binding score characteristics among the selected ligands. The In-silico study aims to find the therapeutic potential of B. diffusa phytoconstituents against ACE-II. Targeting ACE-II also shows an effect against SARS-CoV-2. It can serve as a rationale for designing a drug for patient infected with COVID-19 and associated diseases.
Keywords:
ACE-II protein, COVID-19, Boerhavia diffusa, In-silico molecular docking, ligands-receptor interactionIntroduction
The COVID-19 pandemic is a viral disease. The causative organism of this disease is the SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2). The impaired immune system is the prime factor behind the pathogenesis of COVID-19. The virus has a spherical shape with a diameter of approximately 60 – 140 nm.1 It is a single-stranded RNA virus consisting of a spike, membrane, envelope nucleocapsid, and glycoprotein. The nucleoside protein is associated with the genomic RNA, while other proteins construct protein envelopes around it. Viral infection involves cell penetration and used host cell mechanisms to make replicas of the virus and then release it from the host cell. S1 and S2 subunits of spike protein plays a significant role in regulating virus entry into host cells. The receptor-binding site (RBS) is present in the S1 subunit. It binds with the peptide site (PS) of the angiotensin-converting enzyme-II (ACE-II) protein.2 The S2 subunit facilitates membrane fusion and entangles the spike protein to the virion membrane.3 It is a crucial step that promotes the fusion of the host cell membranes and virus. The spike protein of SARS-CoV-2 acts as a substrate for ACE-II receptors. The virus could infect the cells expressing ACE-II receptors, including alveolar, neuroepithelial, endothelial, renal, macrophages, monocytes, neurons, glial, and intestinal epithelial cells.4 As a result, genetic changes in the ACE-II sequence may alter the molecular interaction of the RBS and PS, which influence both the severity of the disease and host susceptibility to the virus. ACE-II is present on chromosome Xp22 and occupies 39.98 kb of genomic DNA. It has 20 introns and 18 exons. It is a type-I membrane-bound glycoprotein with an 805 amino acid catalytic domain.5 Loop ridges, helices, and some beta sheets cover the ACE-II protein. ACE-II has two domains: a carboxyl-terminal domain that aids receptor binding and an Amino-terminal domain with one zinc metallopeptidase active site. Angiotensin (Ang) is converted into Ang I by renin, whereas Ang I is converted into Ang II by ACE-II. Ang II is responsible for vasoconstriction, hypertension, and cardiac hypertrophy.6 Due to the widespread expression of ACE-II receptors in several organs, which may cause hypertension, diabetes, and cancer are the main risk factors for the progression and prognosis of COVID-19.7-9 Furthermore, the COVID-19 clinical history reveals gender differences in morbidity and mortality. Males are nearly three times more likely to be infected than females.10 Numerous research trials have been performed using synthetic drug molecules as antivirals or immunomodulators.11 Although some drugs have been prescribed for COVID-19 patients, these synthetic compounds have a toxic effect that could cause overstimulation and a decline in cellular functions.12 For instance, 2-DG (2-deoxy-D-Glucose) and hydroxy-chloroquininone have lately been utilized to decrease COVID-19 infection.13 However, it has been noted that they may cause increased melanin, skin rashes, ophthalmic, disorientation, and cardiac damage. Therefore, there is an urgent need to replace synthetic compounds with other phytoconstituents that offer therapeutic support to COVID-19 patients and collaborate to enhance organism health. The phytochemicals extracted from Boerhavia diffusa are classified as alkaloids, rotenoids, phenolics, glycosides, nucleosides, lignans, and steroids.14,15 The root of B. diffusa plant consisting of lignin, liriodendrin (syringaresinol β-D-diglucoside or acanthoside D). It has anti-inflammatory and cardioprotective activity. Liriodendron mitigated sciatic endometriosis-related pain in rats by suppressing the inflammation and regulating the Pl3K/Akt/mTOR signaling pathway.16 In the case of acute lung injury, oral administration of liriodendron causes a significant reduction of pulmonary inflammation in LPS-induced mice.17 Boeravinone B diminished myocardial infarction by lowering oxidative stress in the cardiac tissue by decreasing expressions of caspase-3 & 9, p53, BAX, cyt C, NGAL, TNF, IL-1 & 6, and increasing Bcl-2 expression.18 Eupalitin has anti-neuroinflammation in in-vitro microglial cell lines.19 Several active phytoconstituents of the B. diffusa plant, including liriodendron, boeravinone J, boerhavisterol, bio-quercetin, 2-3-4 beta-ecdysone, kaempferol, biorobin, quercetin, and trans-caftaric acid, inhibited SAR-CoV-2 Main Protease.20 To save money and time, researchers are conducting In-silico methods. The primary object of ongoing pharmaceutical research is the target and lead discovery prediction. To properly understand the pharmacological activity of bioactive compounds, In-silico approaches are combined with other advanced scientific tools to support innovation in health services, academic and industrial research, and development. The In-silico study suggests that phytoconstituents from B. diffusa plants have therapeutic potential in attenuating ACE-II expression. Using phytoconstituents to target ACE-II against SARS-CoV2 could be a rational approach for developing future drugs for COVID and other related diseases.
Experimental
Preparation of ligands – The In-silico studies is crucial in identifying ligands against the target. To be a drug, phytoconstituents must obey Lipinski's rule of five (Supplementary data Table 1). Phytoconstituents of B. diffusa were downloaded in SDF format from the Indian medicinal plants, phytochemistry, and therapeutics (IMPPAT 2.0)21 database, and some phytocompounds were drawn using ChemDraw. All the 3D structures are saved in SDF format. The drug-likeness, physicochemical, lipophilicity, solubility, and pharmacokinetic properties of different phytoconstituents were studied by SwissADME.22 The energy minimization can be done using the UFF algorithm in PyRx software. 2-deoxy-D-Glucose (2-DG) is used as a controlled drug.
Preparation of protein – The 3D structure of the ACE-II protein (code 1R4L) was downloaded from the protein data bank (http://www.rcsb.org) in PDB format.23 The X-ray diffraction of target proteins is used to select them for docking studies. The chosen protein should not have any protein breaks in its whole 3D shape.24 The R-value around 0.2 showed that the selected protein model is efficient for molecular docking studies. The selected protein structure was associated with the ligand (2-acetamido-2-deoxy-β-D-glucopyranose). It was prepared by using Biovia discovery studio software. The ligand and water molecule were removed from the protein, and polar hydrogen was added to the protein and saved in PDB format for docking analysis.
In-silico molecular docking study – In-silico molecular docking predicts the binding affinity of the receptor protein and ligand. The prepared ligands and protein receptor were selected for molecular docking. PyrX software was used to conduct the In-silico docking study. Protein and ligands are saved in PDBQT format after being identified as macromolecules and ligands. The UFF force field was chosen to minimize ligand energy. The PyRx software’s vina wizard tool docks each ligand with the appropriate protein. For docking of ligands X, Y, and Z, a grid box dimension was set to 60. The best interaction was chosen based on the lowest binding energy (Kcal/mol). The lowest binding energy model was chosen and saved as a PDB file. The discovery studio displays the minimum binding energy output model. The Protein-ligand interaction, types of amino acids involved, and types of bonds formed between amino acid residue and ligands were analyzed and saved for further study.
In-silico ADME analysis – The SwissADME (http://www.swissadme.ch) is an available access web tool for Pharmacokinetic ADME ligands studies. This tool provides various physicochemical, pharmacokinetics, lipophilicity, drug-likeness, and water solubility parameters. Lipinski's rule of five gives information on the drug-likeness of orally active compounds. The boiled egg illustration demonstrated whether the ligand could cross the blood-brain barrier and enter the gastrointestinal membrane. The screened phytoconstituents’ absorption, distribution, metabolism, and excretion characteristics were investigated. This study's findings were helpful in the selection of active phytoconstituents.
Analysis of specific cellular pathway – The cellular pathway utilized by coronavirus is obtained from open access source KEGG (https://www.genome.jp/kegg/pathway.html). The pathway involved with the ACE-II receptor and coronavirus spike protein substrate was described in Fig. S1.
Receptor-substrate interaction by STRING network analysis – STRING (Search tool for Retrieval of Interacting Genes) is an online database to predict receptor-substrate interaction and describe their functional activity. The database provides information about a substrate’s biological processes, cellular components, and molecular actions.
Bioactivity score analysis using Molinspiration tool – Molinspiration (http://www.molinspiration.com/cgi-bin/properties) identifies the drug resemblance characteristics of the substance based on different descriptors. Molinspiration tools describe the bioactive score of phytoconstituents against the receptors like protease, GPCR, ion channel, kinase, and proteins. A receptor-substrate complex dynamic behavior finds out by the bioactive score. A bioactive score greater than 0 considers a good score for complexes.
Results and Discussion
The experimental work is based on the execution of an In-silico molecular docking study to predict the Protein-ligand interaction. The binding of phytoconstituents with ACE-II receptors causes the down-regulation of ACE activity. Docking algorithms enforced the phytoconstituents’ stimulating and inhibiting properties with ACE-II receptors, establishing a link between ligand structure and activity.
In the proposed work, more than 50 phytoconstituents were selected from the B. diffusa plant through the IMPPAT database, and some phytoconstituents were drawn using the ChemDraw tool. ACE-II receptor was docked with all the phytoconstituents. The docking interaction and energy minimization scores are given in Table 2. However, β-carotene, repenol, liriodenine, and diffusaroteniod were observed to be the most efficient. The observed binding energy of β-carotene, repenol, liriodenine, and diffusaroteniod with ACE-II receptor is –10.7, –10.3, –10.1, and –10.1 Kcal/mol, respectively. 2-DG was selected as a control for the target ACE-II receptor. 2-DG is the most commonly used synthetic drug against COVID infection. The binding energy of 2-DG against the ACE-II receptor is –5.7 Kcal/mol. The interaction of 2-DG, β-carotene, repenol, liriodenine, and diffusarotenoid is depicted in Fig. 1. The interaction of different amino acid residues of 1R4L with ligands (β-carotene, repenol, liriodenine, and diffusarotenoid, and 2-DG) is given in Table 2. β-carotene interacts with the Van der Waal and Pi-Alkyl bond. Liriodenine interact with Van der Waal, H-bond, Pi-Pi stacked, T-shaped and Pi-alkyl bond. The H-bond observed between the oxygen and nitrogen atom of liriodenine with ARG 518. Liriodendrin interacts with the Van der Waal, H-bond, C-H bond, Pi-Pi T-shaped, alkyl, and Pi-Alkyl. The H-bond observed between the pyranose OH group of Liriodendrin and heteroatom of LYS 562 and GLN 98 amino acids. The Oxygen atom of the bicyclic five-member ring of the liriodenine forms an H bond with LYS 562 and ASP 206 amino acids. Repenol ligand interact with amino acid residue by Van der Waal, H-bond, Pi-Pi stacked, and Pi-anion. The oxygen atom of the carbonyl group and phenolic OH of repenol formed H-bond with ARG 518 and ASP 367 amino acids, respectively. Diffusarotenoid interacts with amino acid residue by Van der Waal, H-bond, C-H bond, Pi-cation & anion, alkyl & Pi-alkyl. The oxygen atom of the carbonyl group and phenolic OH of diffusarotenoid formed H-bond with ARG 518 and PRO 346 amino acids, respectively. The control ligand 2-DG interacts with protein by forming H-bond, Van der Waal, and C-H bond. The OH of 2-DG formed H-bond with TYR 515 and HIS 505 amino acids, respectively. The amino acid residue of 1R4L protein given in Table 2 interacts with 2-DG. Although similar amino acid residue interaction found in B. diffusa plant extract (β-carotene: ARG 273, 514, PHE 504, HIS 374 & 378; Repenol: HIS 374 & 388, GLU 402, PHE 504; diffusarotenoid: ASP 378, GLU 402, HIS 345; liriodenine: ARG 514). It exhibited that this plant extract may have antiviral properties.
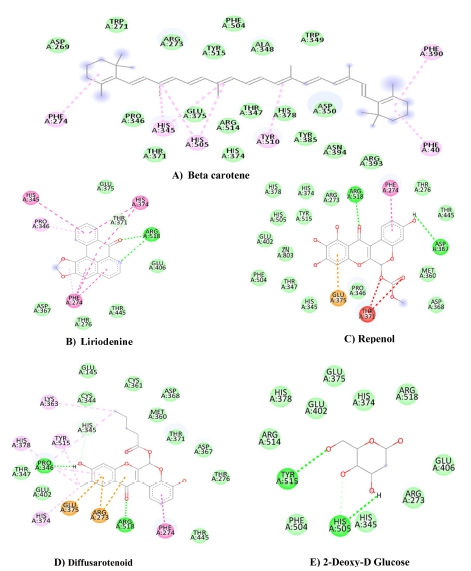
In-silico molecular docking study of selective phytoconstituents of B. diffusa plant with ACE-II protein (code 1R4L).
The binding affinity of phytoconstituents with ACE-II is given in table S1. Around 40 phytoconstituents of the B. diffusa plant have greater and ten phytoconstituents have a lesser affinity than control ligand 2-DG. Some of the phytoconstituents that have a higher affinity as compared to control ligand 2-DG are β-carotene –10.7, repenol –10.3, liriodenine –10.1, diffusarotenoid –10.1, boeravinone B –10, boeravinone F –10, stigma sterol glucoside –10, b-amyrin –10, campesterol glucoside –10, boeravinone A –9.8, stigmas-5 –9.8; It reveals that B. diffusa plant extract has ACE-II inhibitor properties.
The proposed molecular docking studies suggested the characteristics of greater binding affinity of selected phytoconstituents with ACE-II compared to the synthetic inhibitors 2-DG; it shows the therapeutic relevance of the B. diffusa plant in combating COVID-19-associated problems.
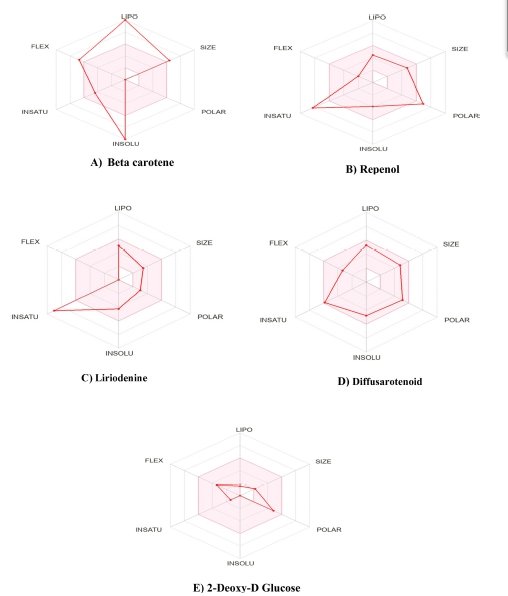
A SwissADME analysis was carried out to identify the drug-likeliness characteristics of screened phytoconstituents. Lipinski et al. proposed various parameters to find the drug-likeness character of orally administered drugs. The colored zone of the radar is the suitable physiochemical area for oral bioavailability. The desired value of the parameters like; molecular weight not more than 500 g/mol, lipophilicity (XLOGP3) should be less than 5, polarity in the range of 20–130 Å, no. of rotatable bonds should be less than 9, and the value of water solubility parameter Log S(ESOL) in the range –6 to 0;25 defining the physiochemical parameters of β-carotene, repenol, liriodenine, and diffusarotenoid showed significant response with control 2-DG (Fig. 2).
Liriodenine, repenol, and diffusarotenoid obey all the Lipinski rules of five, like control 2-DG molecules. It exhibited that it can be orally administered and absorbed by GIT26. In addition, liriodenine, repenol, and diffusarotenoid have 0 violations of Lipinski and an almost similar bioavailability score of 0.55 as 2-DG. The phytoconstituents given in Table 1 had a feature to be drug molecules; however, a molecule having a greater affinity than 2-DG is found to be the better option for drug discovery. The bioavailability and absorption of the selected candidate can be improved in the future by formulation developments.
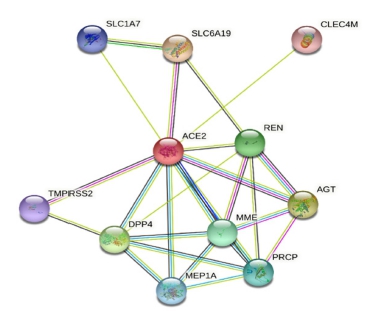
Fig. 3 exhibited the interaction of ACE-II receptors with endogenous molecules detected by the STRING target network (string-db.org). ACE-II interacts with various protein given in Fig. 3. Dipeptidyl peptidase 4 (DPP4) is a cell surface glycoprotein receptor involved in T-cell activation. Lysosomal pro-X carboxypeptidase (PRCP) inactivates angiotensin and bradykinin. Transmembrane protease serine 2 (TMPRS S2) is a serine protease that promotes coronavirus uptake. C-type lectin domain family 4 members (CLEC 4 M) mediate the pathogen’s endocytosis. Renin (REN) is an endopeptidase that produces angiotensin I from angiotensinogen. Angiotensinogen (AGT) regulates blood pressure, electrolyte homeostasis, and body fluid through the renin-angiotensin system (RAS).
The physiochemical parameters in Table 1 represent selected phytoconstituents showing drug-likeness characters similar to the 2-DG. The lipophilicity parameters of phytoconstituents presented in Table 1 exhibit positive values, which indicates a higher lipid affinity than 2-DG. The order of lipophilicity of the selected compounds is β-carotene > diffusarotenoid > repenol > liriodenine > 2-DG.
KEGG analysis was performed to predict the clinical significance of the identified target and the involvement of ACE-II. Around ten targets, including those in Fig. 3, were identified via string analysis27. These targets were mainly associated with the clinical activity of ACE-II. The KEGG cell signaling pathways related to COVID-19 and the renin-angiotensin system are shown in Fig. S1. Furthermore, the viral spike protein was received by ACE-II and NRP1 in the COVID-19 pathway and transferred into the cell via endocytosis or membrane fusion. Ang I and II enter the host cell via MAS1 and AT1R, followed by ADAM17, TNFR, EGFR, and TLR2/4.
Further, we identified the bioactivity scores to determine the potency of phytoconstituents (Table 3). The result reveals that liriodenine has a more excellent enzyme inhibitor score than the control drug, 2-DG. Bioactivity scores of phytoconstituents and the control drug 2-DG were compared, indicating that the proposed phytoconstituents might be used as an alternative to the treatment of COVID-19.
Identifying selective targets leads to determining mechanistic pathways involved in coronavirus integration into the biological system and related chronic diseases. Coronavirus entry into the human genome is aided by ACE-II.28 The phytoconstituents of B. diffusa have shown explicit findings against coronavirus. Molecular docking of the phytoconstituents with protein has provided good binding affinity, and the study is presented to assess ligands in-vitro as well as in-vivo study.
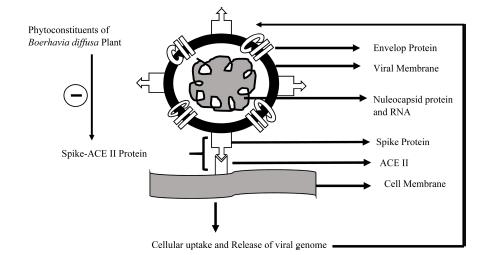
Out of all phytoconstituents, liriodenine has exhibited good affinity, obeys Lipinski’s drug-likeness, and has a more enzyme inhibitor score. Downstream to molecular mechanism, ACE-II protein is also associated with other cellular mechanisms like cell growth, vasodilation, and amyloid-β metabolic activities29. We propose phytoconstituents of the B. diffusa plant as ACE-II inhibitors to combat COVID-19 (Fig. 4). However, such findings need further confirmation by utilizing in-vitro experiments and molecular dynamics simulation.
In conclusion, herbal or medicine plants have the potential to treat clinical illnesses. The proposed study investigated the clinical effectiveness of some phytoconstituents extracted from the B. diffusa plant. The findings are expected to pique the scientific community's interest in drug development against COVID-19, for which no specific drug has been discovered using herbal products. The forecast prospect of the proposed study is to explore the antiviral activity of selected phytoconstituents-rich plant extract by performing in-vitro cell line study. The safety and efficacy will ensure by in-vivo studies. The approach of the study is to investigate herbal bioactive compounds for COVID-19 and associated diseases moreover, similar research can be done with other medicinal plants. Furthermore, our research is being expanded to look at the effects of other diseases that may be contributing to the progression of the coronavirus.
Acknowledgments
This work was supported by a grant (3-66/2021-CCRAS/Admn/IMR/1631) from the Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, Government of India. The authors also thankful to DG, CCRAS and Director, National Ayurveda Research Institute for Panchakarma, Thrissur, India for providing continuous support and encouragement.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Porter, J.; Blau, E.; Gharagozloo, F.; Martino, M.; Cerfolio, R.; Duvvuri, U.; Caceres, A.; Badani, K.; Bhayani, S.; Collins, J.; Coelho, R.; Rocco, B.; Wiklund, P.; Nathan, S.; Parra-Davila, E.; Ortiz-Ortiz, C.; Maes, K.; Dasgupta, P.; Patel, V. BJU Int. 2020, 126, 225–234.
[https://doi.org/10.1111/bju.15105]

-
Hashemi, S. M. A.; Thijssen, M.; Hosseini, S. Y.; Tabarraei, A.; Pourkarim, M. R.; Sarvari, J. Arch. Virol. 2021, 166, 2089–2108.
[https://doi.org/10.1007/s00705-021-05070-6]

-
Jackson, C. B.; Farzan, M.; Chen, B.; Choe, H. Nat. Rev. Mol. Cell Bio. 2022, 23, 3–20.
[https://doi.org/10.1038/s41580-021-00418-x]

-
Gu, W.; Gan, H.; Ma, Y.; Xu, L.; Cheng, Z. J.; Li, B.; Zhang, X.; Jiang, W.; Sun, J.; Sun, B.; Hao, C. Virol. J. 2022, 19, 49.
[https://doi.org/10.1186/s12985-022-01783-5]

-
Singh, H.; Choudhari, R.; Nema, V.; Khan, A. A. Microb. Pathog. 2021, 150, 104621.
[https://doi.org/10.1016/j.micpath.2020.104621]

-
Bhushan, S.; Xiao, Z.; Gao, K.; Mao, L.; Chen, J.; Ping, W.; Hong, W.; Zhang, Z. Curr. Probl. Cardiol. 2022, 48, 101162.
[https://doi.org/10.1016/j.cpcardiol.2022.101162]

-
Cheng, H.; Wang, Y.; Wang, G. Q. J. Med. Virol. 2020, 92, 726–730.
[https://doi.org/10.1002/jmv.25785]

-
Bhowmick, N. A.; Oft, J.; Dorff, T.; Pal, S.; Agarwal, N.; Figlin, R. A.; Posadas, E. M.; Freedland, S.; Gong, J. Endocr. Relat. Cancer 2020, 27, R281–R292.
[https://doi.org/10.1530/ERC-20-0165]

-
Zaki, N.; Alashwal, H.; Ibrahim, S. Diabetes Metab. Syndr. 2020, 14, 1133–1142.
[https://doi.org/10.1016/j.dsx.2020.07.005]

-
Peckham, H.; de Gruijter, N. M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L. R.; Rosser, E. C.; Webb, K.; Deakin, C. T. Nat. Commun. 2020, 11, 6317.
[https://doi.org/10.1038/s41467-020-19741-6]

-
Nugraha, R. V.; Ridwansyah, H.; Ghozali, M.; Khairani, A. F.; Atik, N. Evid. Based Complement. Alternat. Med. 2020, 2020, 2560645.
[https://doi.org/10.1155/2020/2560645]

- Creagh, S.; Warden, D.; Latif, M. A.; Paydar, A. Clin. Radiol. Imaging. J. 2018, 2, 000116.
-
Huang, Z.; Chavda, V. P.; Vora, L. K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z. S. Front. Pharmacol. 2022, 13, 899633.
[https://doi.org/10.3389/fphar.2022.899633]

-
Gaur, P. K.; Rastogi, S.; Lata, K. Phytomed. Plus 2022, 2, 100260.
[https://doi.org/10.1016/j.phyplu.2022.100260]

-
Maurya, R.; Boini, T.; Misro, L.; Thulasi, R.; Singh, R. Curr. Hypertens. Rev. 2023, 19, 1–17.
[https://doi.org/10.2174/1573402119666230221084859]

- Gong, J.; Xue, L.; Wei, M.; Han, W.; Jing, S. Arch. Med. Sci. 2021, in press.
-
Yang, L.; Li, D.; Zhuo, Y.; Zhang, S.; Wang, X.; Gao, H. Inflammation 2016, 39, 1805–1813.
[https://doi.org/10.1007/s10753-016-0416-1]

- Chen, Y.; Peng, L.; Shi, S.; Guo, G.; Wen, H. J. Cell. Mol. Med. 2021, 25, 6403–6416.
- Kumar, J. R.; Varadaraju, K. R. Eur. J. Mol. Clin. Med.2021, 8, 2207–2216.
-
Rutwick Surya, U.; Praveen, N. Virusdisease. 2021, 32, 46–54.
[https://doi.org/10.1007/s13337-021-00683-6]

- Vivek-Ananth, R. P.; Mohanraj, K.; Sahoo, A. K.; Samal, A. bioRxiv 2022, 496609.
-
Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 42717.
[https://doi.org/10.1038/srep42717]

-
Towler, P.; Staker, B.; Prasad, S. G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N. A.; Patane, M. A.; Pantoliano, M. W. J. Biol. Chem. 2004, 279, 17996–18007.
[https://doi.org/10.1074/jbc.M311191200]

-
Upreti, S.; Prusty, J. S.; Pandey, S. C.; Kumar, A.; Samant, M. Mol. Divers. 2021, 25, 1795–1809.
[https://doi.org/10.1007/s11030-020-10159-2]

- Lawal, B.; Liu, Y. L.; Mokgautsi, N.; Khedkar, H.; Sumitra, M. R.; Wu, A. T. H.; Huang, H. S. Biomedicines 2021, Biomedicines, 92.
-
Andrews, C. W.; Bennett, L.; Yu, L. X. Pharm. Res. 2000, 17, 639–644.
[https://doi.org/10.1023/A:1007556711109]

-
Wicik, Z.; Eyileten, C.; Jakubik, D.; Simões, S. N.; Martins Jr, D. C.; Pavão, R.; Siller-Matula, J. M.; Postula, M. J. Clin. Med. 2020, 9, 3743.
[https://doi.org/10.3390/jcm9113743]

-
Luo, J.; Lu, S.; Yu, M.; Zhu, L.; Zhu, C.; Li, C.; Fang, J.; Zhu, X.; Wang, X. Gene 2021, 768, 145325.
[https://doi.org/10.1016/j.gene.2020.145325]

-
Rezaei, M.; Ziai, S. A.; Fakhri, S.; Pouriran, R. J. Cell. Physiol. 2021, 236, 2430–2442.
[https://doi.org/10.1002/jcp.30041]