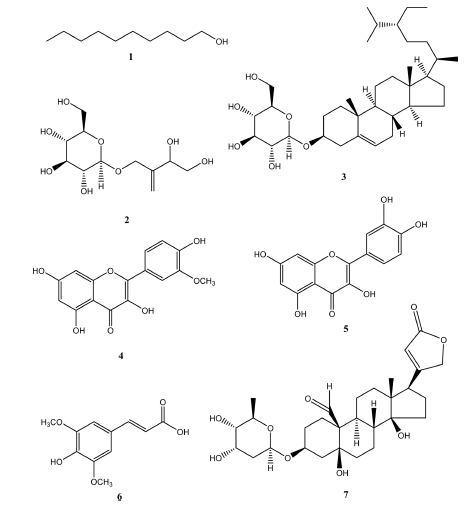
Anti-influenza Compounds Isolated from Descurainia sophia Seeds
Abstract
Descurainia sophia seeds methanol extract showed significant anti-influenza activity and we tried to isolate anti-influenza compounds from the D. sophia extract. D. sophia seeds were extracted with 80% methanol and fractionated with n-hexane, ethyl acetate, CHCl3 and n-butanol. The anti-influenza activity of each fraction was assessed using sulforhodamine B (SRB) method in A549 cells, human-derived lung cancer cells. The ethyl acetate and CHCl3 fractions showed the most potent anti-influenza activity. Seven compounds were isolated from CHCl3 fraction and identified 1-decanol (1), 2-(3,4-dihydroxy-2-methylenebutoxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (2), daucosterol (3), isorhamnetin (4), quercetin (5), sinapic acid (6), and helveticoside (7) by spectroscopic data such as UV, IR, 1H-NMR, 13C-NMR and mass spectroscopy. Anti-influenza activities of isolated compounds were evaluated using SRB method in A549 cells. Compounds 3, 4 and 7 had significant anti-influenza activity in a dose-dependent manner.
Keywords:
Descurainia sophia seed, anti-influenza, daucosterol, isorhamnetin, helveticosideIntroduction
Influenza viruses have three serotypes, such as A, B and C. Stereotype B and C were well known to be infected only in humans, and serotype A was known to infect mammals and poultry only. However, recently, there has been cases of human infection due to the recombination of gene of sterotype A influenza virus. Hemagglutinin (HA) and neuraminidase (NA) were distributed on the surface of the Influenza A virus. HA played an important role for the virus to infiltrate into the host cells. And NA enabled to cut the bond of HA and glycoprotein located in the host cell surfaces and influenza virus to be released from the host cell.1 Influenza viruses are RNA viruses and have the character of rapid growth and easy variation.2 There are two types of antigen mutations that cause mutations in protein on the surface of influenza viruses. Antigen drift is an antigenic mutation that occurs due to the gradual accumulation of amino acid deletions or substitutions along the point mutations in the molecules of the HA or NA antigen in the same subtype. Antigen shift is an antigenic mutation that spans the subtype of the antigenically unrelated influenza A virus, a mutation that completely changes the amino acid structure of the hemagglutinin or NA antigen protein.3 Therefore, it is also important to prevent this virus from spreading to other individuals after infection, and it is also important to prevent transmission from cells in the body to cells.4 Well-known antiviral drugs include neuraminidase inhibitors oseltamivir, zanamivir, amantadine and rimantadine, which inhibit the M2 protein. Neuraminidase inhibitors are effective against both influenza A and B, while M2 protein inhibitors are effective against influenza A only.5 However, recently, the number of cases of influenza viruses resistant to neuraminidase inhibitors has been increasing, and development of new anti-influenza drugs has become necessary.
Recently, much research for isolation of components from medicinal plants for the development of anti-influenza drugs have been widely and actively conducted. Herbal medicine has traditionally been used to ensure the safety aspect, and it can be expected that a compound treatment effect of various ingredients can be expected rather than the effect of one ingredient.6 In the course of search for anti-influenza activities in various natural product extracts, the total methanolic extract of the Descurainia sophia was exerted anti-influenza activity in our assay system. In this study, we presently tried to isolate the anti-influenza active compounds from the seeds of D. sophia.
D. sophia had been used as traditional medicine for a long time in Korea, China and Japan. D. sophia seeds has been used to treat various diseases, such as cough and asthma and showed cardiotonic, purgative, febrifuge, antipruritic, antihelmintic, expectorant, astringent and litholytic activity.7,8 In addition, recent studies have been reported that D. sophia had the antioxidant activity, skin whitening activity, anti-cancer activity against the breast and uterine cancer cells, anti-constipation activity and anti-inflammation activity. 9-13D. sophia seeds contained a variety of compounds such as benzyl, allyl, propenyl-isothiocyanate, cardiac glycosides, flavonoids, phenols, lignans, sulphur glycoside, lactones and coumarins.14-16
Anti-influenza activity of D. sophia seeds was evaluated by using sulforhodamine B (SRB) method through A549 cell, a human-derived lung cancer cell.17 SRB is a bright pink amino-zantine dye with two sulfonic groups and is often used for protein staining. Under a slightly acidic condition, SRB binds to the protein's basic amino acid residues in the cell, indicating a linear correlation with cell density. The advantage of the SRB method is that it can detect the anticancer effect against human cancer cells in a shorter time than the MTT assay or the XTT assay and provides a stable end point that is less susceptible to changes in the surrounding environment and is hardly affected by the intermediate metabolites.18 This analysis relies on the ability to bind cellular protein components and measure total biomass, which is confirmed by measuring the cytopathic effect (CPE).19
In this study, we presently isolated seven compounds from methanol extract of D. sophia by activity guided isolation and identified their structures by spectroscopic data. Moreover, we also evaluated the anti-influenza activity of these compounds by SRB method in A549 cells.
Experimental
Plant materials – The seeds of Descurainia sophia were purchased from medicinal herbs market called by Miryon Herbal Medicine (Seoul, Korea). Specimens of samples (NO. CJ128M) has been deposited in the Kangwon National University Natural Product laboratory (Chuncheon, Korea).
General – Methanol, n-hexane, CHCl3, ethyl acetate and n-butanol for extraction and partition were purchased from SK Chemical (Seoul, Korea). Silica gel 60 F254 plates used for TLC were obtained from Merck (Germany). The spots on TLC were visualized by soaking with anisaldehyde-H2SO4 followed by heating. Silica gel (70-230 mesh, Merck, Germany) and sephadex LH-20 (bead size 25-100 mm, Sigma, USA) was used for stationary phase of column chromatography. HPLC grade solvents, such as water, acetonitrile and methanol were purchased from J.T. Baker (USA). TFA (trifluoroacetic acid) was purchased from DAE JUNG in Korea. UV spectra detecting lamp for reading the TLC plates is UV GL-58 (U.S.A). Jeol JNM-ECZ400S/L1 (400 MHz) (Japan) and Bruker Avance Ⅱ 600 (600 MHz) (Germany) were used for the NMR data. Bruker Autoflex speed TOF/TOF (Germany) was used to measure the mass spectroscopy of chemical compounds.
Cell culture and in vitro test regents – Influenza A/PUERTO RICO/8 virus was obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). A549 cells were purchased from ATCC (Rockville, MD, USA) and maintained in Dulbacco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, FBS, and DMEM were purchased from Gibco BRL (Invitrogen Life Technologies, Karlsruhe, Germany). TPCK-Trypsin was purchased from Pierce (Thermo Fisher Scientific, Rockford, IL, USA). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Osetamivir carboxylate used as positive control, was purchased from Sigma-Aldrich (St. Louis, MO, USA)
Extraction and isolation – Dried seeds of Descurainia sophia (6 kg) were extracted with 80% methanol for 90 min with nine times at 25oC by ultrasonication-assisted extraction. The extracted solution was evaporated to remove solvents and made total methanolic extract. The methanolic extract of D. sophia was suspended in distilled water. Water suspended extract solution was portioned with n-hexane, CHCl3, ethyl acetate and n-butanol in a polarity order. As a result, each fraction yielded at the amounts of n-hexane fraction (74.66 g), CHCl3 fraction (4.07 g), ethyl acetate fraction (2.98 g) and n-butanol fraction (20.81 g), respectively. Among them, CHCl3 fraction showed the most potent anti-influenza activity and we tried to isolate the active ingredients from CHCl3 fraction. The CHCl3 fraction was subjected to MPLC system (SANP cartridge KP-Sil 340g, Biotage) and eluted with a gradient of CHCl3:methanol (99:1→0:100, v/v) to obtain ten subfractions named as A - J. Anti-influenza activity of subfraction A to J were evaluated and subfraction B exerted most potent anti-influenza activity. Subfraction B was subjected to Prep-HPLC (acetonitrile: water = 30:70 → 100:0, v/v) to give three subfraction (Ba-Bc). The fraction Ba solution was concentrated in a rotary vacuum evaporator and suspended on methanol. Compound 1 (15.0 mg) was obtained in a crystalline form and washed thoroughly with methanol. Compound 2 (65.1 mg) was obtained from the fraction Bb by continuous Prep-HPLC (acetonitrile:water = 30:70 → 100:0, v/v). The fraction Bc solution was concentrated in a vacuum concentrator and suspended on methaol. Compound 3 (42.2 mg) was obtained in the form of crystals and washed thoroughly with methanol. Subfraction E also showed significant anti-influenza activity. Subfraction E was loaded into MPLC system (SANP cartridge KP-Sil 340 g, Biotage) and eluted with a gradient of n-hexane: ethyl acetate (99:1 → 0:100, v/v) to obtain seven subfractions named as a - g. Fraction Eb was subjected to preparative HPLC (acetonitrile:water = 30:70 → 100:0, v/v). Compound 4 (29.6 mg) was obtained as a powder form from HPLC of fraction Eb. Compound 5 (59.6 mg) was obtained as a crystal form from HPLC of fraction Eb. Fraction Ef was loaded to preparative HPLC. The mobile phase condition is acetonitrile:water = 40:60 → 100:0 (v/v) and five subfractions were obtained (Ef1-Ef5). Compound 6 (40.8 mg) was obtained from fraction Ef2 by preparative HPLC (acetonitrile:water = 20:80 → 100:0, v/v). Compound 7 (42.6 mg) was obtained from fraction Ef4 by preparative HPLC (acetonitrile:water = 30:70 → 100:0, v/v).
in vitro anti-influenza activity assay - Anti-influenza activity was measured by confirming the degree of reduction of CPE using the SRB method. A549 cells were seeded at a cell concentration of 2 × 104 cell/well of a 96 well plate and cultivation for 24 hours. The following day, a diluted virus suspension containing 1 μg/mL of TPCK trypsin was added to apiece well and the selected concentration of fraction added. Untreated virus-infected cells were used as a virus control, and uninfected and untreated cells were used as a cell control. After cultivation for 2 days, cells were washed with PBS, added with ice-cold 70% acetone, and incubated at -20oC for 30 min. The acetone was removed and the 96 well plate was dried in a drying oven for 30 min and then 0.4% (w/v) SRB in 1% acetic acid was supplemented to apiece well for 30 min at the room temperature. Subsequently, the SRB was removed and the plate was washed with 1 % acetic acid before oven drying. And then, 1 day of drying, the SRB was solubilized in a 10 mM non-buffered Tris solution and the absorbance was read at 540 nm using a VERSA max microplate reader (Molecular Devices, Palo Alto, CA, USA) with a standard absorbance at 620 nm.
Statistical analysis – The results of cellular viability of A539 cells was presented as mean ± SD, respectively. All experimental results were statistically analyzed using one-way ANOVA and Tukey’s post hoc test with IBM SPSS Statistics software V26 (IBM, Armonk, NY, US) and Microsoft Excel software (Microsoft, US). The differences between the experimental groups were significant at p < 0.05, 0.01 and 0.001 level, respectively.
Results and Discussion
As a series of our search for anti-influenza active compounds of medicinal plants, the methanolic extract of the seeds of D. sophia showed significant anti-influenza activity in the A549 cells against influenza A/Puerto Rico/8 virus (Fig. 1). However, it has been confirmed that the methanol extract of D. sophia does not exhibit cytotoxicity even at high concentrations (Fig. 2) The seeds of D. sophia has been well known as the traditional oriental medicines for treatments of cough and asthma. In this research, we tried to present anti-influenza active compounds of D. sophia seeds against influenza A/Puerto Rico/8 virus as measured in vitro. The 6.0 kg of D. sophia seeds were extracted with 80% methanol and total methanolic extract (221.54 g) was obtained. Methanolic extract was sequentially fractionated by solvent polarity and obtained n-hexane (74.66 g), CHCl3 (4.07 g), ethyl acetate (2.98 g) and n-butanol (20.81 g) fractions. Anti-influenza activity against the influenza A/Puerto Rico/8 virus of the four fractions of methanol extract of D. sophia were evaluated using by the SRB method to monitor changes in CPE induced by viral infection. Among them, the chloroform fraction showed most potent significant anti-influenza activity against influenza A/Puerto Rico/8 virus in A549 cells in a dose dependent manner (Fig. 3). Then, we tried to isolate active compounds from chloroform fraction by using various of chromatographic techniques such as open-column chromatography with stationary phase of silica gel or sephadex LH-20, MPLC and preparative HPLC. At first, we divided into subfraction for chloroform fraction to subject open column chromatography and obtained ten subfractions named fraction A to J. The anti-influenza activities of subfractions A to J were also evaluated using by the same method. Subfraction B and E exerted the strongest anti-influenza activities and we tried to isolate active components from the subfraction B and E. As a result, we isolated three compounds from subfraction B including alkyl alcohol and sterol, and four compounds from subfraction E including flavonoid, sterol and phenylpropanoid. These compounds were identified as 1-decanol (1), 3,4-dihydroxy-2-methylenebutyl β-D-glucopyranoside (2), daucosterol (3), isorhamnetin (4), quercetin (5), sinapic acid (6) and helveticoside (7), respectively (Fig. 4). Their structures were identified by the comparison of their spectroscopic data such as 1H and 13C NMR, IR and Mass spectroscopy to reference data.20-24 Among them, compounds 3, 4 and 7 showed significant anti-influenza activities at the concentrations ranging from 1.0 μM to 100.0 μM in a dose dependent manner (Table 1). These compounds might be the candidate of new medicine to treat influenza.
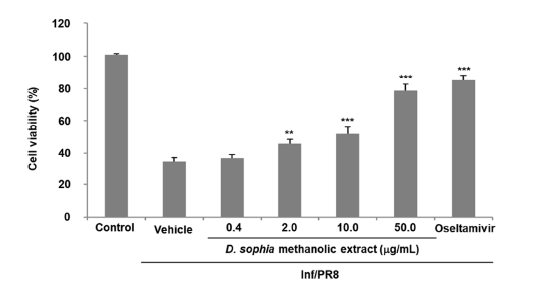
Anti-influenza activity of methanolic extract of Descurainia sophia against (0.4, 2.0, 10.0 and 50.0 μg/mL) against influenza A/Puerto Rico/8 virus induced cell death of A549 cells. Data expressed as mean ± the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001 versus the vehicle-treated group.
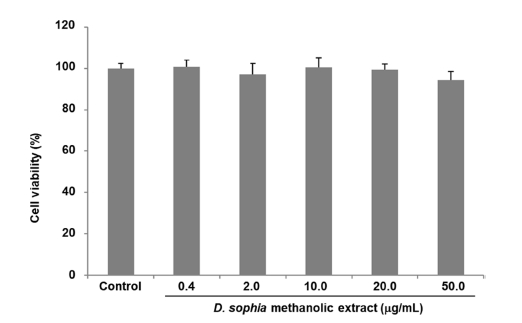
Cytotoxicity activity of methanolic extract of D. sophia (0.4, 2.0, 10.0, 20.0 and 50.0 μg/mL) in the A549 cells. Data expressed as mean ± the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001 versus the vehicle-treated group.
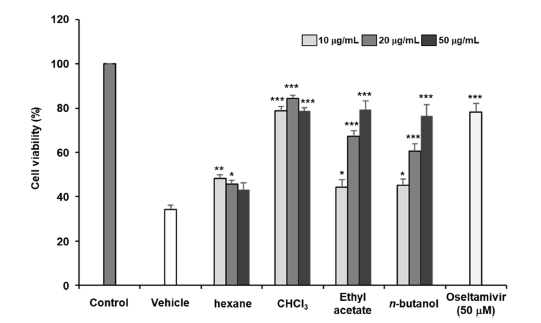
Anti-influenza activity of hexane, chloroform, ethyl acetate and n-butanol fractions of methanolic extract of D. sophia (10.0, 20.0 and 50.0 μg/mL) against influenza A/Puerto Rico/8 virus induced cell death of A549 cells. Data expressed as mean ± the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001 versus the vehicle-treated group.

Anti-influenza activity of compounds isolated from the CHCl3 fraction of D. sophia methanolic extract against influenza A/Puerto Rico/8 virus induced cell death in A549 cells
In recent studies, compound 3 (daucosterol) has been reported that it effectively inhibited the growth of various cancer cells, and showed anti-oxidative activity, anti-inflammation, and anti-candidiasis.25-29 Isorhamnetin (4) also has been reported that it showed cardiovascular and cerebrovascular protection, antioxidant, anti-inflammatory neuroprotective and anti-cancer activity.30 Helveticoside (7) is a kind of iridoid glycoside and showed various pharmacological activities including sedative and anxiolytic activity, anti-inflammatory, antioxidant, neuroprotective and anti-cancer activity.31
The present data suggested that the seeds of D. sophia may have anti-influenza therapeutic potential and three compounds 3, 4 and 7 may be active components of D. sophia seeds. As we noted above, there has been in vitro result of anti-influenza constituents from D. sophia. Thus, further elucidation of mechanisms of action and studies of in vivo biological activities should be performed to develop anti-influenza drugs using the seeds of D. sophia.
In conclusion, seven compounds including 1-decanol (1), 3,4-dihydroxy-2-methylenebutyl β-D-glucopyranoside (2), daucosterol (3), isorhamnetin (4), quercetin (5), sinapic acid (6) and helveticoside (7) were isolated from chloroform fraction of D. sophia of 80% methanolic extract. The anti-influenza active compounds of D. sophia extract were also identified as three of the compounds – daucosterol (3), isorhamnetin (4) and helveticoside (7). The elucidation of mechanisms of actions and in vivo anti-influenza activities of three compounds were required in the further study to develop anti-influenza drug from D. sophia seeds and three compounds.
References
-
Slemons, R. D.; Johnson, D. C.; Osborn, J. S.; Hayes, F. Avian Dis. 1974, 18, 119-124.
[https://doi.org/10.2307/1589250]

-
Zheng, W.; Tao, Y. J. FEBS Lett. 2013, 587, 1206-1214.
[https://doi.org/10.1016/j.febslet.2013.02.048]

-
Hong, E. H.; Song, J. H.; Kang, K. B.; Sung, S. H.; Ko, H. J.; Yang, H. J. Biomol. Ther (Seoul). 2015, 23, 345-349.
[https://doi.org/10.4062/biomolther.2015.019]

-
Jiang, W. Y. Trends Pharmacol. Sci. 2005, 26, 558-563.
[https://doi.org/10.1016/j.tips.2005.09.006]

-
Bent, S. J. Gen. Intern. Med. 2008, 23, 854-859.
[https://doi.org/10.1007/s11606-008-0632-y]

-
Firenzuoli, F.; Gori, L. Evid. Based Complement. Alternat. Med. 2007, 4, 37-40.
[https://doi.org/10.1093/ecam/nem096]

-
Sun, K.; Li, X.; Li, W.; Wang, J.; Liu, J.; Sha, Y. Chem. Pharm. Bull (Tokyo). 2004, 52, 1483-1486.
[https://doi.org/10.1248/cpb.52.1483]

- Askari, H.; Enayati, N.; Ahmadian-Attari, M. M.; Bakhtiyari, M.; Alireazei, A. Iran. J. Pharm. Res. 2021, 20, 40-52.
-
Xu, W.; Chu, K.; Li, H.; Chen, L.; Zhang, Y.; Tang, X. Molecules 2011, 16, 10029-10045.
[https://doi.org/10.3390/molecules161210029]

- Choi, H.; Ahn, S.; Lee, B. G.; Chang, I.; Hwang, J. S. Pigment Cell Res. 2005, 18, 439-446.
- Khodarahmi, E.; Asghari G. H.; Hassanzadeh, F.; Mirian, M.; Khodarahmi, G. A. Res. Pharm. Sci. 2015, 10, 169-176.
-
Nimrouzi, M.; Sadeghpour, O.; Imanieh, M. H.; Shams Ardekani, M.; Salehi, A.; Minaei, M. B.; Zarshenas, M. M. Iran. J. Pediatr. 2015, 25, e425.
[https://doi.org/10.5812/ijp.425]

-
Lee, Y. J.; Kim, N. S.; Kim, H.; Yi, J. M.; Oh, S. M.; Bang, O. S.; Lee, J. Arch. Pharm. Res. 2013, 36, 536-541.
[https://doi.org/10.1007/s12272-013-0066-x]

-
Sun, K.; Li, X.; Li, W.; Liu, J. M.; Wang, J. H.; Sha, Y. Nat. Prod. Res. 2006, 20, 519-522.
[https://doi.org/10.1080/14786410500045309]

-
Sun, K.; Li, X.; Liu, J. M.; Wang, J. H.; Li, W.; Sha, Y. J. Asian Nat. Prod. Res. 2005, 7, 853-856.
[https://doi.org/10.1080/1028602042000204072]

- Mohamed, N. H.; Mahrous, A. E. Rec. Nat. Prod. 2009, 3, 58-67.
-
Kim, B. Y.; Lee, J.; Kim, N. S. BMC Genomics 2015, 16, 713.
[https://doi.org/10.1186/s12864-015-2192-y]

-
Vichai, V.; Kirtikara, K. Nat. Protoc. 2006, 1, 1112-1116.
[https://doi.org/10.1038/nprot.2006.179]

-
Song, J.; Yeo, S. G.; Hong, E. H.; Lee, B. R.; Kim, J. W.; Kim, J.; Jeong, H.; Kwon, Y.; Kim, H.; Lee, S.; Park, J. H.; Ko, H. J. Biomol. Ther (Seoul). 2014, 22, 41-46.
[https://doi.org/10.4062/biomolther.2013.108]

-
Cano, R.; Yus, M.; Ramón, D. J. Tetrahedron 2011, 67, 8079-8085.
[https://doi.org/10.1016/j.tet.2011.08.063]

- Zaman, M. K.; Ali, M.; Siddiqui, A. W.; Rafiullah, M. R. M. J. Saudi Chem. Soc. 2005, 9, 161-170.
-
Faizi, S.; Ali, M.; Saleem, R.; Irfanullah.; Bibi, S. Magn. Reson. Chem. 2001, 39, 399-405.
[https://doi.org/10.1002/mrc.855]

-
Webster, R. G.; Bean, W. J.; Gorman, O. T.; Chambers, T. M.; Kawaoka, Y. Microbiol. Rev. 1992, 56, 152-179.
[https://doi.org/10.1128/mr.56.1.152-179.1992]

-
Bloom, D. E.; Black, S.; Rappuoil, R. Proc. Natl. Acad. Sci. USA. 2017, 114, 4055-4059.
[https://doi.org/10.1073/pnas.1701410114]

-
Salimi, M.; Ardestaniyan, M. H.; Mostafapour Kandelous, H.; Saeidnia, S.; Gohari, A. R.; Amanzadeh, A.; Sanati, H.; Sepahdar, Z.; Ghorbani, S.; Salimi, M. Cell Prolif. 2014, 47, 172-179.
[https://doi.org/10.1111/cpr.12090]

-
Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K. P. Sci. Rep. 2017, 7, 3418.
[https://doi.org/10.1038/s41598-017-03511-4]

- Thanh, T. B.; Duc, L. V.; Thanh, H. N.; Tien, V. N. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 79-84.
-
Choi, J. N.; Choi, Y. H.; Lee, J. M.; Noh, I. C.; Park, J. W.; Choi, W. S.; Choi, J. H. Nat. Prod. Res. 2012, 26, 2340-2343.
[https://doi.org/10.1080/14786419.2012.654608]

-
Lee, J. H.; Lee, J. Y.; Park, J. H.; Jung, H. S.; Kim, J. S.; Kang, S. S.; Kim, Y. S.; Han, Y. Vaccine 2007, 25, 3834-3840.
[https://doi.org/10.1016/j.vaccine.2007.01.108]

-
Gong, G.; Guan Y. Y.; Zhang Z. L.; Rahman, K.; Wang, S. J.; Zhou, S.; Luan, X.; Zhang, H. Biomed. Pharmacother. 2020, 128, 110301.
[https://doi.org/10.1016/j.biopha.2020.110301]

-
An, N.; Sun, Y.; Ma, L.; Shi, S.; Zheng, X.; Feng, W.; Shan, Z.; Han, Y.; Zhao, L.; Wu, H. Arch. Med. Res. 2020, 51, 224-232.
[https://doi.org/10.1016/j.arcmed.2020.02.007]