Macagigantin A, A New Flavonoid from Macaranga gigantea (Rchb.f & Zoll.) Mull.Arg
Abstract
A new flavonol, macagigantin A (1), and three known flavonols (2–4) were isolated from Macaranga gigantea leaves. The structure of macagigantin A was fully assigned by 1D and 2D NMR, UV, and high-resolution mass spectra data. The cytotoxic activity of 1–4 was evaluated against 4T1, P-388, and HeLa cells. Compound 1 showed potent activity against 4T1 cells with an IC50 value of 1.18 μg/mL, and compound 3 showed moderate activity against P-388 cells (IC50 value of 2.54 μg/mL).
Keywords:
Macagigantin A, Flavonoid, Macaranga gigantea, CytotoxicIntroduction
The genus Macaranga is a pioneer plant usually found in the secondary forests and a member of the Euphorbiaceae family. The leaves of Macaranga are empirically used for treating fever, wounds, coughs, and cancer. The leaves of M. recurvata were traded for cancer treatment by the Dayak community in Kalimantan, Indonesia.1-2 The secondary metabolites commonly found in the leaves of the Macaranga plant include flavonoids, terpenoids, and stilbenoids, and exhibit biological activities such as antimalaria, antioxidant, anti-inflammatory, antibacterial, and anticancer.3-8 Flavonols and flavanones are the major flavonoids in the Macaranga plant. Kaempferol and quercetin derivatives with terpenyl chains on both aromatic nuclei are characteristic of the genus Macaranga. 9-12
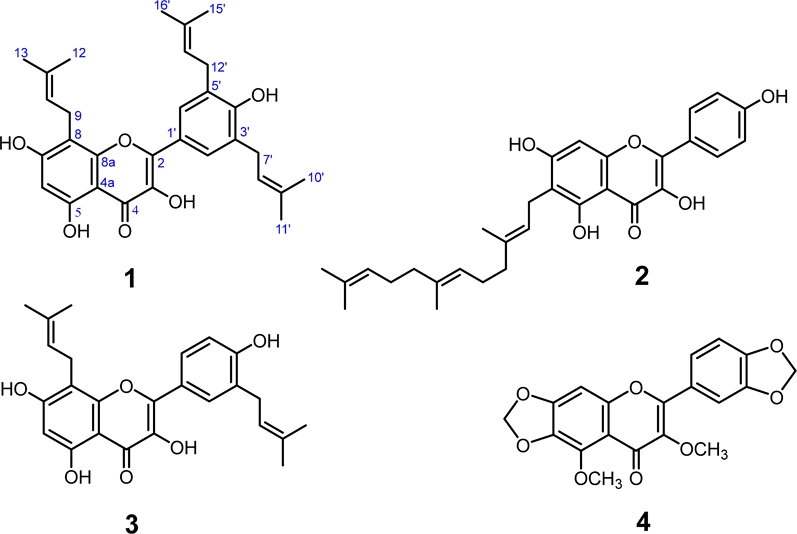
Macaranga gigantea (Rchb.f & Zoll.) Mull. Arg is one of the plant species that first grew in damaged forests. M. gigantea makes open areas quickly become secondary forests. M. gigantea is a type of plant that grows throughout the Indonesian archipelago. Four flavonol derivatives were isolated from the leaves of M. gigantea, including a new compound, macagigantin A (1), and three known flavonols, macagigantin (2), broussoflavonol F (3), and meliternatin (4). The cytotoxic of flavonols 1–4 was evaluated against breast cancer cells (4T1), leukemia (P-388), and cervical cells (HeLa).
Experimental
General experimental procedures – The instrumentation used in determining the structure of flavonols 1–4 used a UV spectrophotometer, mass spectrometer, and NMR spectrometer operating 400 MHz. The λmax of flavonoids 1–4 was measured with a Shimadzu UV-Vis spectrophotometer series 1800 in the λ 200–400 nm. The chemical formulas 1–4 were determined using a high-resolution ESIMS spectrometer (Waters-LCT Premier XE). The chemical shifts (δH and δC) of 1–4 were measured with an NMR JEOL ECA-400 spectrometer, operating 400 MHz (1H NMR) and 100 MHz (13C NMR). Silica gel 60, Sephadex LH-20, and PF254 were used as the stationary phase in the gravity column chromatography (CC) and chromatotron.
Plant materials – The leaves of M. gigantea with no. Specimen DMG-20200509 was gathered from Pijor Koling Village, Southeast Padangsidempuan, North Sumatra, Indonesia, in May 2020. Dr. Nuraina identified the materials specimen at Herbarium Universitas Andalas ANDA, Padang, Indonesia.
Extraction and isolation – The dried leaves of M. gigantea (1.5 kg) were extracted with hexane by maceration at room temperature for 24 hours (4 L, two times) to produce a thick hexane extract (130 g). Furthermore, extraction with 90% EtOH and partitioned with ethyl acetate obtained a viscous EtOAc extract (12 g). The separation of the EtOAc extract by silica gel CC, eluting with hexane-EtOAc 7:3 v/v to obtain fractions A (2.1 g) and B (3.1 g). The Sephadex LH-20 CC of fraction A (2.1 g) with MeOH afforded subfractions A1 and A2. The purification of fraction A2 (735 mg) by silica gel chromatotron, eluting with hexanediisopropyl ether (19:1 to 4:1 v/v) to give 1 (5 mg), 2 (31 mg), 3 (12 mg), and 4 (14 mg).
Macagigantin A (1) – Yellow solid; UV (MeOH) λmax (log ε): 220 (4.54), 256 (4.40), 270 (4.28), and 355 nm (4.19); IR (KBr) νmax: 3456, 1630, 1542, and 1445 cm–1; For the NMR spectral data, see Table 1; HRESIMS m/z [M+H]+ calculated for C30H35O6 491.2434, found 491.2453.
Cytotoxic activity – The cytotoxic activity of 1–4 against human cervical cells (HeLa), leukemia (P-388), and human breast cells (4T1) were assessed by the MTT assay according to the experiment previously.9-11 HeLa, P-388 and 4T1 cells were cultured in the RPMI-1640 medium containing 10% FBS at 37oC flowed with 5% CO2 for 48 h. The Hela, P-388 and 4T1 cells were added compounds 1–4 in the 96-well, incubated at 37oC and flowed with 5% CO2 for 24 h. The active compound’s ability to kill cancer cells was evaluated by the microplate reader spectrometer at λ 590 nm.13-16 Doxorubicin is used as the positive control for the cytotoxic assay.
Result and Discussion
Four flavonol derivatives were isolated from M. gigantea leaves, including a new flavonol, macagigantin A (1), and three known compounds, macagigantin (2), broussoflavonol F (3), and meliternatin (4). The NMR spectra of compounds 2–4 are identical to the chemical shifts with the same M. gigantea and Melicope glabra compounds.17-18
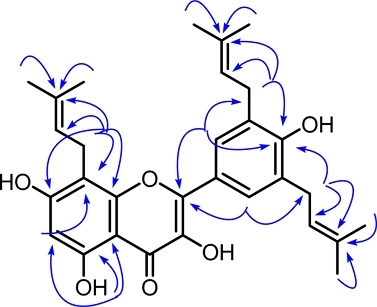
Macagigantin A (1) was obtained as a yellow solid, showing the chemical formula C30H35O6 at the ion peak [M+H]+ m/z 491.2453 (calculated mass: 491.2434) by high-resolution mass spectrum. The UV spectrum of 1 showed the maximum absorption at 220 (4.54), 256 (4.40), 270 (4.28), and 355 (4.19) nm characteristics for flavonol moiety.3 The FT-IR spectrum of macagigantin A, showing the functional group that consists of a hydroxy (3456 cm-1), aromatic C=C (1455 and 1542 cm-1), and C-O-C ether groups (1176 cm-1). The 1H NMR spectrum of macagigantin A (Table 1) exhibited two singlets proton of two aromatic units (A and B rings), a signal at δH 6.34 (1H, s, H-6) for a 1,2,3,4,5 pentasubstituted benzene system (A ring) and a resonance of δH 8.00 (2H, s, H-2'/6') for a symmetrically of 1,3,4,5 tetrasubstituted benzene system (B ring). The proton signal of the hydrogen-bonded of hydrogen group showed at δH 12.11 (1H, s, 5-OH), an isoprenyl chain [a vinylic, δH 5.36 (1H, t, J = 7.3 Hz, H-10, δC 123.1), a methylene, δH 3.56 (2H, d, J = 7.1 Hz, H-9, δC 22.2), two methyls, δH 1.63 (3H, s, H-12, δC 18.1), δH 1.79 (3H, s, H-13, δC 25.9)], and a symmetrically of isoprenyl chain [a vinylic, δH 5.38 (2H, t, J = 7.4 Hz, H-8'/13', δC 122.8), methylene, δH 3.44 (4H, d, J = 7.3 Hz, H-7'/12', δC 29.3), two methyls, δH 1.73 (6H, s, H-10'/15', δC 17.9), δH 1.75 (6H, s, H-11'/16', δC 25.8)]. The 13C NMR spectrum of macagigantin A (Table 1) showed 23 signals from 30 carbons based on the HRESIMS spectrum. Among them, two oxygenated carbons [δC 149.6 (C-2), δC 137.5 (C-3)], four oxy-aryls [δC 162.0 (C-7), δC 159.8 (C-5), δC 154.9 (C-8a), δC 155.2 (C-4')], and one carbonyl (δC 177.3, C-4) characteristic for a kaempferol derivative.3 The HMBC correlations (Fig. 2) described the isoprenyl chain in the kaempferol skeleton. Long-range correlation of the HMBC spectrum, the hydrogen bond of the hydroxy group at δH 12.11 shows a correlation with δC 103.7 (C-4a), δC 159.8 (C-5), and δC 98.7 (C-6). An isolated aromatic proton at δH 6.34 (H-6) correlated to C-4a, C-5, δC 162.0 (C-7), and δC 107.1 (C-8), indicating an isoprenyl chain bonded at C-8. The methylene proton at δH 3.56 (H-9) from the part of the isoprenyl chain correlated to C-7, C-8, δC 154.9 (C-8a), δC 123.1 (C-10), and δC 132.1 (C-11) also supporting the presence of the isoprenyl chain at C-8. The HMBC spectrum, correlations of a symmetric aromatic proton at δH 8.00 (H-2'/6') to δC 149.6 (C-2), δC 128.0 (C-2'/6'), δC 155.2 (C-4'), and δC 29.3 (C-7´/12´) revealed a symmetric isoprenyl chain at C-3'/5'. The signal of methylene from a symmetric isoprenyl chain at δH 3.44 (H-7'/12') correlated to C-2'/6', C-4' δC 122.8 (C-8'/13'), and δC 133.8 (C-9'/14') supporting that a symmetrically of the isoprenyl chain at C-3'/5'. Based on the spectra data above, the structure of macagigantin A (1) is 8,3',5'-triisoprenylquercetin.
The cytotoxicity of flavonols 1–4 against Hela, P-388, and 4T1 cells using the MTT assay by the colorimetric method at 590 nm. Investigation of flavonols 1–4 against HeLa, P-388, and 4T1 cells were evaluated using MTT assay by the colorimetric at 590 nm. Compound 1 showed high activity towards 4T1 cells (IC50 value 1.18 μg/mL) and weak activity against HeLa and P-388 cells (IC50 = 7.12 and 5.98 μg/mL) (Table 2). Compound 3 exhibited moderate activity towards P-388 cells (IC50 value 2.54 μg/mL) and very weak toward HeLa and 4T1 cells (IC50 = 5.60 and 6.42 μg/mL). Compound 2 exhibited very weak towards P-388 cells (IC50 value 6.45 μg/mL) and inactive toward HeLa and 4T1 cells (IC50 = 5.60 and 6.42 μg/mL). Compounds 1 and 3 show the presence of the isoprenyl chain at C-8, and ring B was revealed to increase cytotoxic against three cancer cells.13-15
Acknowledgments
This project was supported by Hibah Riset Mandat Kolaborasi Mitra Luar Negeri, Universitas Airlangga, 2020, No. 782/UN3.15/PT/2021.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Tjahjandarie, T. S.; Tanjung, M.; Saputri, R. D.; Nadar, P. B.; Aldin, M. F.; Marliana, E.; Permadi, A. Nat. Prod. Sci. 2019, 25, 244–247.
[https://doi.org/10.20307/nps.2019.25.3.244]

-
Tanjung, M.; Hakim, E. H.; Elfahmi.; Latip, J.; Syah, Y. M. Nat. Prod. Commun. 2012, 7, 1309–1310.
[https://doi.org/10.1177/1934578X1200701013]

-
Tanjung, M.; Juliawaty, L. D.; Hakim, E. H.; Syah, Y. M. Fitoterapia 2018, 126, 74–77.
[https://doi.org/10.1016/j.fitote.2017.10.001]

-
Tanjung, M.; Hakim, E. H.; Syah, Y. M. Chem. Nat. Compd. 2017, 53, 215–218.
[https://doi.org/10.1007/s10600-017-1955-x]

-
Peresse, T.; Jezequel, G.; Allard, P. M.; Pham, V. C.; Huong, D. T. M.; Blanchard, F.; Bignon, J.; Levaique, H.; Wolfender, J. L.; Litaudon, M.; Roussi, F. J. Nat. Prod. 2017, 80, 2684–2691.
[https://doi.org/10.1021/acs.jnatprod.7b00409]

-
Klausmeyer, P.; Van, Q. N.; Jato, J.; McCloud, T. G.; Beutler, J. A. J. Nat. Prod. 2010, 73, 479–481.
[https://doi.org/10.1021/np9006348]

- Aldin, M. F.; Tjahjandarie, T. S.; Saputri, R. D.; Tanjung, M. Nat. Prod. Sci. 2021, 27, 45–48.
-
Yoder, B.; Cao, S.; Norris, A.; Miller, J. S.; Ratovoson, F.; Razafitsalama, J.; Andriantsiferana, R.; Rasamison, V. E.; Kingston, D. G. I. J. Nat. Prod. 2007, 70, 342–346.
[https://doi.org/10.1021/np060484y]

-
Pailee, P.; Sangpetsiripan, S.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Tetrahedron 2015, 71, 5562–5571.
[https://doi.org/10.1016/j.tet.2015.06.058]

-
Segun, P. A.; Ogbole, O. O.; Ismail, F. M. D.; Nahar, L.; Evans, A. R.; Ajaiyeoba, E. O.; Sarker, S. D. Fitoterapia 2019, 134, 151–157.
[https://doi.org/10.1016/j.fitote.2019.02.019]

- Aminah, N. S.; Kristanti, A. N.; Tanjung, M. J. Chem. Pharm. Res. 2014, 6, 688–692.
-
Marliana, E.; Astuti, W.; Kosala, K.; Hairani, R.; Tjahjandarie, T. S.; Tanjung, M. Asian J. Chem. 2018, 30, 795–798.
[https://doi.org/10.14233/ajchem.2018.21004]

-
Tjahjandarie, T. S.; Tanjung, M.; Saputri, R. D.; Rahayu, D. O.; Gunawan, A. N. I.; Aldin, M. F. Nat. Prod. Res. 2021, 35, 5637–5642.
[https://doi.org/10.1080/14786419.2020.1821016]

-
Saputri, R. D.; Retnowati, R.; Supratman, U.; Tjahjandarie, T. S.; Tanjung, M. Nat. Prod. Res. 2023, 37, 197–203.
[https://doi.org/10.1080/14786419.2021.1960524]

-
Tanjung, M.; Tjahjandarie, T. S.; Aldin, M. F.; Mardhiyyah, S.; Maqfiroh, I.; Saputri, R. D.; Ahmat, N. Nat. Prod. Sci. 2023, 29, 38–41.
[https://doi.org/10.20307/nps.2023.29.1.38]

-
Tanjung, M.; Rachmadiarti, F.; Saputri, R. D.; Tjahjandarie, T. S. Nat. Prod. Res. 2018, 32, 1062–1067.
[https://doi.org/10.1080/14786419.2017.1378215]

-
Saputri, R. D.; Tjahjandarie, T. S.; Tanjung, M. Nat. Prod. Sci. 2018, 24, 155–158.
[https://doi.org/10.20307/nps.2018.24.3.155]

-
Tanjung, M.; Hakim, E. H.; Mujahidin, D.; Hanafi, M.; Syah, Y. M. J. Asian Nat. Prod. Res. 2009, 11, 929–932.
[https://doi.org/10.1080/10286020903302315]