Anti-pemphigus and Anti-atopic Potentials of Lycopi Herba Extract with MKK3 Inhibitory Activity in Human Keratinocyte HaCaT Cells
Abstract
Pemphigus is autoimmune blistering disease associated with autoantibodies (such as desmoglein 3 antibody) directed against the cell surface of keratinocytes, thereby loss of cell-cell adhesion of keratinocytes. The pathogenesis of pemphigus is currently thought to be mediated by direct inhibition via autoantibodies and subsequent signal transduction involving p38 mitogen activated protein kinase (MAPK) pathway. Several studies have reported both mitogen-activated protein kinase kinase 3 (MKK3) and MKK6 are required for full activation of p38, and we recently reported the activation of MKK3 in pemphigus skin tissue, but not MKK6, suggesting that MKK3 could be a potential therapeutic target for pemphigus vulgaris. Here, we found that AK23 IgG (desmoglein 3 antibody) induced the phosphorylation of MKK3 in human keratinocyte HaCaT cells, and treatment of MKK3-inhibiting Lycopi Herba extract (ELH) with MKK3 kinase IC50, 12.25 g/mL significantly inhibited AK23-induced fragmentation of HaCaT cell sheets in a dose-dependent manner without any cytotoxicity. Additionally, ELH exhibited anti-atopic activity. In conclusion, MKK3 could play an important role in blister formation in pemphigus, and the MKK3 inhibition by herbal extracts such as ELH could be a possible therapeutic strategy for treating patients with pemphigus as well as atopic dermatitis.
Keywords:
Pemphigus, MKK3, Lycopi Herba, Lycopus lucidus TurczIntroduction
Pemphigus refers to a group of autoimmune blistering diseases of skin and mucous membranes associated with autoantibodies directed against the cell surface of keratinocytes, resulting in the loss of cell-cell adhesion of keratinocytes. Pemphigus can be divided into four major types: vulgaris, foliaceus, paraneoplastic, and IgA pemphigus.1 Of them, classical types of pemphigus are pemphigus vulgaris (PV) and pemphigus foliaceus (PF). In both PF and PV, the pathogenic IgG antibodies primarily target desmoglein (Dsg), trasmembrane glycoproteins of desmosomes. The target antigen of PV is Dsg 3, while the PF target antigen is Dsg 1.2–4
In pemphigus, the disruption of cell-cell adhesion has been currently thought to be mediated by autoantibodies as well as by subsequent signal transduction triggered by antibody binding. The role of p38 mitogen activated protein kinase (MAPK) pathway has been well-established in pathogenesis of pemphigus. It plays an important role in cellular response to various infectious and environmental stimuli as well as osmotic/oxidative stress.5 Activation of p38 is found in in vitro studies using PV serum IgG and mAbs, and in skin samples of patients with pemphigus, thereby making p38 a promising drug therapy target. However, p38 not only have four different isoforms (α, β, γ, δ), but it is also involved in various cellular processes. Therefore, targeting p38 using p38 inhibitors may lead to the off-target effects and systemic toxicities.6
The classical p38 MAPK pathway involves the activation of MEKK4, which then activates MKK3, MKK4, or MKK6. These kinases subsequently phosphorylate p38 MAPK at Thr180 and Tyr182, initiating signaling. MKK3 and MKK6, with roughly 80% amino acid similarity, are primary contributors to the activation of p38.7 Previous studies have reported both MKK3 and MKK6 are required for full activation of p38 MAPK in MKK3/MKK6 knock out mice.8 In addition, both MKK3 and MKK6 are important regulators of p38 and activated in the synovium of rheumatoid arthritis (RA) patients.9 We identified the relevance of both MKK3 (not MKK6) and p38 to pemphigus in the previous "study, here hypothesized that the inhibition of MKK3 by natural resources could provide the benefits in the treatment of pemphigus.10
Lycopi Herba (Lycopus lucidus Turcz.), commonly known as Chinese bugleweed, is a traditional medicinal plant with a long history of use in East Asian medicine for its perceived therapeutic benefits, particularly in blood circulation improvement, anti-inflammatory applications, and immune modulation. It is frequently used in Chinese medicine to address various ailments, including inflammation, cardiovascular diseases, and menstrual disorders, highlighting its potential in diverse therapeutic areas.11–15 The bioactive properties of Lycopi Herba are attributed to its rich chemical profile, including phenolic compounds, flavonoids, triterpenoids, polysaccharides, and essential oils.11–15 However, the effect of the extract of Lycopi Herba (ELH) on the pemphigus has not been reported. Here, we found that the activation of MKK3 in human keratinocyte HaCaT cells and anti-pemphigus activity of ELH with the inhibitory potential against MKK3 kinase activity.
Experimental
Preparation of ELH ─ The ELH (CA04-062) used in this study was sourced from the Korea Plant Extract Bank at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea), where a voucher specimen (PBC-394) is archived. Dried and powdered Lycopi Herba (110 g) was combined with 1 L of 95.0% ethyl alcohol (GR grade) and subjected to 30 extraction cycles at room temperature using an ultrasonic extractor (40 kHz, 1500W; SDN-900H, SD-Ultrasonic Co.). The extract was filtered (Qualitative Filter No. 100, Hyundai Micro Co.) and dried under reduced pressure, yielding 6.19 g of ELH.
Cell culture ─ The immortalized human keratinocyte HaCaT cells (AddexBio, CA, USA) were cultured in DMEM/low glucose with 10% fetal bovine serum and 1% antibiotic-antimycotic solution at 37°C and 5% CO2. All cell culture materials were obtained from HyClone (UT, USA).
Western blot analysis ─ HaCaT cells (5 × 105 cells/well) were seeded in a 6-well plate, incubated for 24 h, and then treated with 5 μg/mL of anti-Dsg 3 monoclonal antibody clone AK23 IgG (MBL, Japan; following referred to as AK23) for indicated time. Cells were washed with PBS, lysed with RIPA buffer (ELPIS biotech, Korea) buffer containing the protease inhibitor cocktail (ThermoFisher Scientific, MA, USA) and the phosphatase inhibitor cocktail (ThermoFisher Scientific) on ice, and centrifuged 13,000 rpm for 30 min at 4℃. The amount of proteins in the supernatant was quantified by Pierce BCA Protein Assay Kit (ThermoFisher Scientific). Proteins (10 μg) were resolved by 5-20% gradient gel (ATTO, Tokyo, Japan), electrophoresis at 130 V for 90 min and transferred to polyvinylidene difluoride (ATTO). The transferred membranes were blocked with TBST (Tris-buffered saline, 0.1% Tween 20) with 5% BSA at room temperature for 1 h, and the membranes were incubated with the indicated antibodies for overnight at 4℃. All antibodies were purchased form Cell Signaling Technology (MA, USA), and GAPDH was used as the internal control. After three washes with TBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 2 hours at room temperature. Following another three TBST washes, the HRP signal was detected using ECL western detection reagents (Millipore Corporation, MA, USA) on a WSE-6100 LuminoGraph (ATTO).
MKK3 kinase activity assay ─ MKK3 kinase activity was carried out in Eurofins (France). Briefly, the human recombinant MKK3 was incubated with 25 mM Tris/HCl, pH 7.5, 0.2 mM EGTA, 0.5 mM sodium orthovanadate, 0.5 mM β-glycerophosphate, 0.01% Triton X-100, 1% glycerol, 10 mM DTT, 0.5 mg/mL MBP, 20 µg/mL unactive form of the human p38 recombinant protein (substrate of MKK3), 10 mM Magnesium acetate and 155 mM [γ-33P-ATP]. The reaction began with the addition of the Mg/ATP mixture. After a 120-minute incubation at room temperature, phosphoric acid was added to reach a concentration of 0.5%, halting the reaction. A 10 µL sample of the mixture was then placed onto a P30 filtermat, washed four times in 0.425% phosphoric acid for 4 minutes each, and rinsed once in methanol before drying and scintillation counting.
Cell viability assay ─ HaCaT cells (4 × 103 cells/well) were seeded in a 96-well plate, incubated for 24 h, and then treated with ELH for 1 day. Cell viability was assessed in triplicate using Cell Counting Kit-8 (Dojindo Molecular Technologies, ML, USA) following the manufacturer’s instructions. Absorbance was measured at 450 nm using HIDEX sense microplate reader (Hidex, Finland). Dose response curves showing cell viability were generated using GraphPad Prism 5 (GraphPad Software Inc., CA, USA).
Dispase-based fragmentation assay ─ HaCaT cells (5 × 105 cells/well) were seeded in a 24-well plate, incubated for 24 h, and then treated with 5 μg/mL of AK23 without or with ELH for 24 h. Then, cells were washed with prewarmed PBS and incubated with 150 μL of Hanks buffered saline solution (HBSS; Gibco) containing Dispase Ⅱ solution (2.4 U/mL; Sigma) for 20 min at 37℃. After the addition of 200 μL of HBSS, cell monolayer was exposed to mechanical stress by pipetting them 15 times with a 1 mL pipette for shearing the monolayer. Subsequently, cells were stained with MTT (2 mg/mL; Sigma) for 20 min. The resulting fragments were counted and images were captured under the microscope. Dissociation assay were performed in triplicate, and statistical differences were analyzed using Student’s t-test. A value of p < 0.05 was considered significant.
Enzyme-linked immunosorbent assay ─ HaCaT cells (1 × 106 cells/well) were seeded in a 6-well plate with 10% FBS containing DMEM. And then, the cells were pretreated with natural product ELH for 1 h and then treated with TNF-α and INF-γ (5 ng/mL) for 24 h. Thymus and activation-regulated chemokine (TARC) levels in the cell supernatant were quantified through a sandwich enzyme-linked immunosorbent assay (ELISA), utilizing a human CCL17/TARC quantikine ELISA kit (R&D Systems) in accordance with the manufacturer’s instructions.
Results and Discussion
To investigate the effect of AK23 on the phosphorylation of MKK3 in HaCaT cells, cells were treated with 5 μg/mL of AK23 for different time intervals (0, 5, 15, and 30 minutes) and the protein levels of phosphorylated MKK3 (p-MKK3), total MKK3, and GAPDH were analyzed by Western blot. As shown in Fig. 1, the level of p-MKK3 showed significant changes following treatment with AK23. The intensity of the p-MKK3 bands increased starting from the 5-minute treatment, continuing to increase at 15 and 30 minutes, suggesting that AK23 could promote the phosphorylation of MKK3. MKK3 and GAPDH used as a loading control exhibited stable expression across all samples.
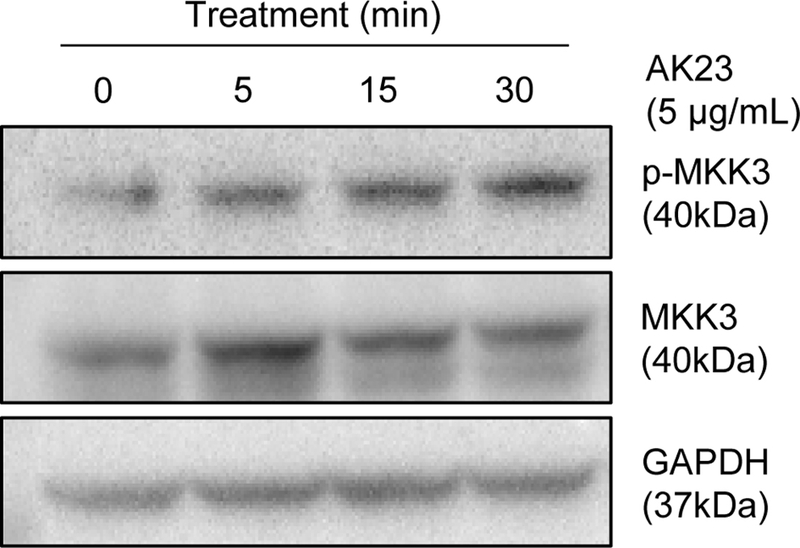
Effect of AK23 on MKK3 phosphorylation in HaCaT cells. Activation of MKK3 was confirmed by western blot analysis.
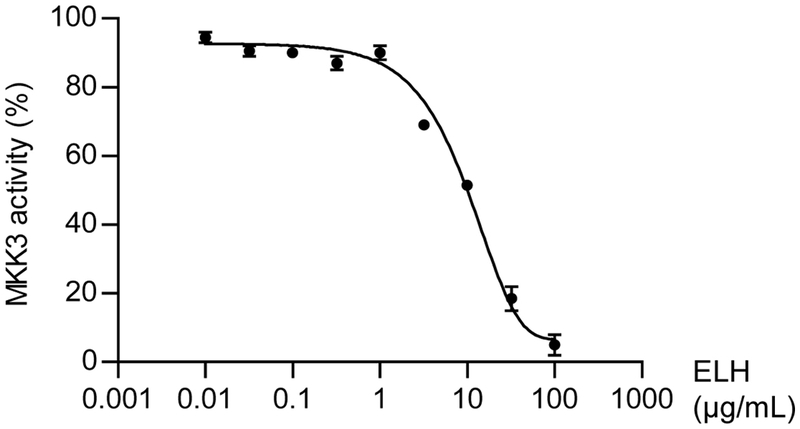
Herbal extracts in library were screened and finally ELH was identified to inhibit MKK3 kinase activity (data not shown). As shown in Fig. 2, ELH inhibited MKK3 activity in a dose-dependent manner with IC50 value, 12.25 μg/mL.
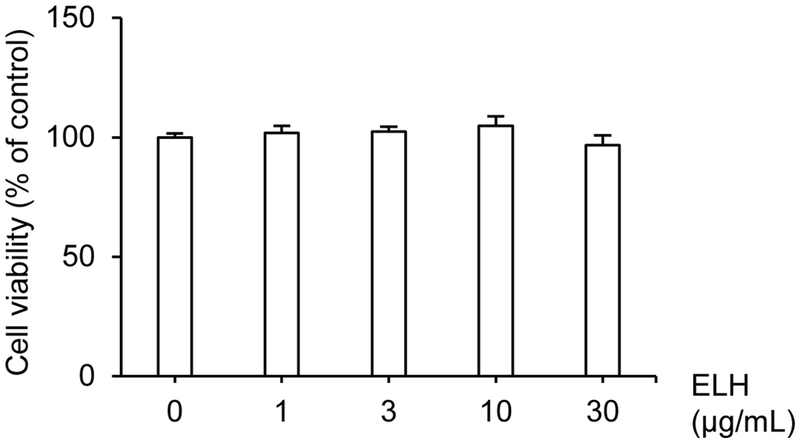
Before evaluating whether ELH could exhibit anti-pemphigus activity in HaCaT cells, the non-toxic concentration ranges of ELH was determined to exclude the possibility that ELH could induce cell detachment due to cytotoxicity. HaCaT cells were treated with various concentrations of ELH and cultured for 1 day, followed by measuring cell viability using the CCK8 assay. ELH did not show any cytotoxicity upto 30 μg/mL (Fig. 3). Therefore, the effect of ELH on pemphigus was evaluated upto 30 μg/mL by the dispase-based fragmentation assay that has been reported as the representative in vitro pemphigus model using monolayers of cells such as HaCaT cells. As shown in Fig. 4, AK23 treatment in HaCaT cell monolayers strongly increased number of fragments indicating the loss of intercellular adhesion that is the main characteristic of autoimmune-blistering disease of the skin such as pemphigus. However, the AK23-induced fragmentation was significantly inhibited by ELH in a dose-dependent manner, suggesting that ELH could exhibit the anti-pemphigus via its potential to inhibit MKK3 kinase activity.
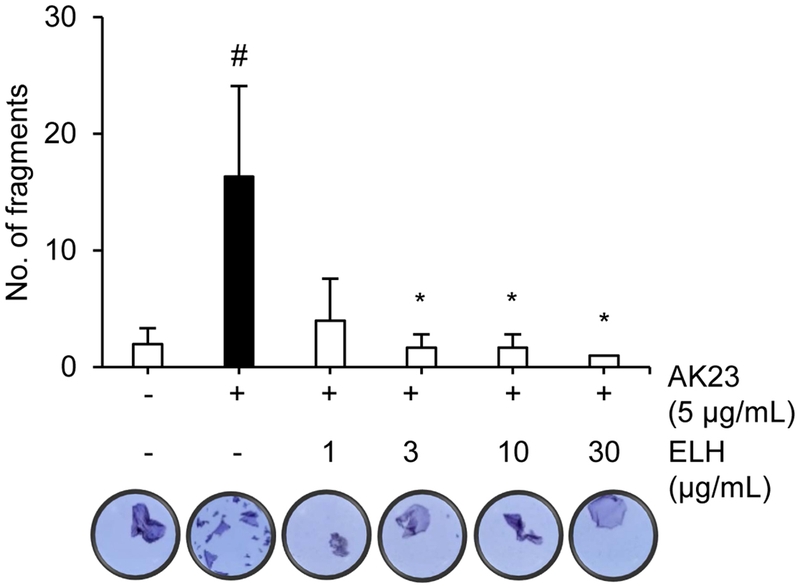
Anti-pemphigus activity of ELH in HaCaT cells. Antipemphigus activity of ELH was evaluated by the dispase-based fragmentation assay. The resulting fragments were counted and images were captured under the microscope. #p < 0.05 (vs. the control); *p < 0.05 (vs. AK23-treated)
Pemphigus and atopic dermatitis are associated with shared pathogenic mechanism.16,17 In fact, anti-atopic effects and their underlying mechanisms of Lycopi Herba has been reported in several cell lines including HaCaT cells, as well as DNCB-induced animal models.18 Also, the MKK3-mediated atopic dermatitis in HaCaT cells has been recently reported.19 Therefore, the anti-atopic activity of ELH with the inhibitory potential against MKK3 kinase activity was further evaluated. The pro-inflammatory cytokine TARC was strongly induced in HaCaT cells treated with TNF-α and IFN-γ, but the pre-treatment of ELH was significantly attenuated the TNF-α/IFN-γ-induction of TARC in a dose-dependent manner, suggesting that MKK3-inhibiting ELH could exhibit anti-atopic activity as well as anti-pemphigus activity (Fig. 5).
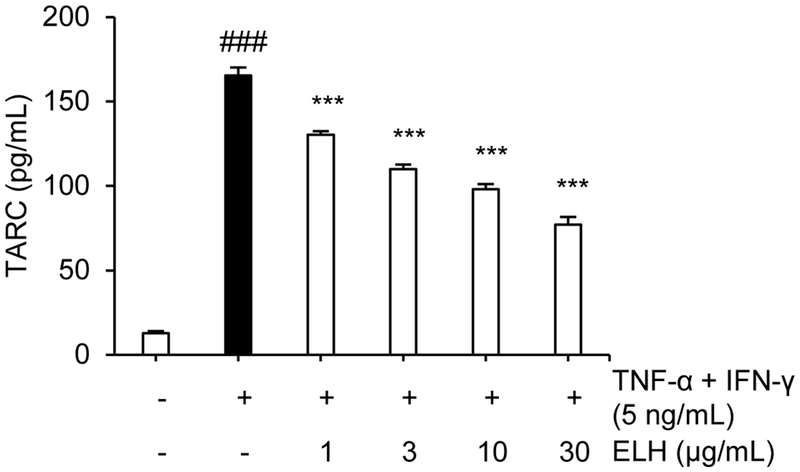
Anti-atopic activity of ELH in HaCaT cells. Anti-atopic activity of ELH was evaluated by measuring the protein level of human CCL17/TARC. ###p < 0.001 (vs. the control); ***p < 0.001 (vs. AK23-treated)
The role of MKK3 as a critical target in various diseases, including atopic dermatitis, has recently been in the spotlight in the field of drug discovery. In previous study, the relevance of MKK3 to pemphigus has been reported and its therapeutic potential as druggable target for treating skin disorders. In the context of discovering natural product to novel therapeutics substances for skin disorders, we selected Lycopi Herba (Lycopus lucidus Turcz.) as an MKK3 inhibitor through the target-based screening and confirmed its anti-pemphigus activity as well as its anti-atopic dermatitis activity. In conclusion, MKK3 could play a pivotal role in blister formation in pemphigus, and its inhibition by the medicinal plant extracts such as Lycopi Herba, could provide novel therapeutic strategy for treating patients with skin disorders such as pemphigus and atopic dermatitis. Further study would focus on the identification of active compounds from Lycopi Herba to determine the specific components responsible for MKK3 inhibition and anti-pemphigus activity.
Acknowledgments
This study was supported by Korea Research Institute of Chemical Technology (KK2031-10, SI2131-10, SI2231-10, KK2331-10 and KK2431-10).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
REFERENCES
-
Malik, A. M.; Tupchong, S.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. Medicina (Kaunas) 2021, 57, 1080.
[https://doi.org/10.3390/medicina57101080]

-
Heupel, W.-M.; Zillikens, D.; Drenckhahn, D.; Waschke, J. J. Immunol. 2008, 181, 1825–1834.
[https://doi.org/10.4049/jimmunol.181.3.1825]

-
Schlögl, E.; Radeva, M. Y.; Vielmuth, F.; Schinner, C.; Waschke, J.; Spindler, V. Front. Immunol. 2018, 9, 858.
[https://doi.org/10.3389/fimmu.2018.00858]

-
Schmitt, T.; Waschke, J. Front. Med. 2021, 8, 701809.
[https://doi.org/10.3389/fmed.2021.701809]

-
Mao, X.; Sano, Y.; Park, J. M.; Payne, A. S. J. Biol. Chem. 2011, 286, 1283–1291.
[https://doi.org/10.1074/jbc.M110.172874]

-
Mao, X.; Li, H.; Sano, Y.; Gaestel, M.; Park, J. M.; Payne, A. S. J. Invest. Dermatol. 2014, 134, 68–76.
[https://doi.org/10.1038/jid.2013.224]

-
Mavropoulos, A.; Orfanidou, T.; Liaskos, C.; Smyk, D. S.; Billinis, C.; Blank, M.; Rigopoulou, E. I.; Bogdanos, D. P. Autoimmun. Rev. 2013, 12, 580–590.
[https://doi.org/10.1016/j.autrev.2012.10.019]

-
Lu, H. T.; Yang, D. D.; Wysk, M.; Gatti, E.; Mellman, I.; Davis, R. J.; Flavell, R. A. EMBO J. 1999, 18, 1845–1857.
[https://doi.org/10.1093/emboj/18.7.1845]

-
Chabaud-Riou, M.; Firestein, G. S. Am. J. Pathol. 2004, 164, 177–184.
[https://doi.org/10.1016/S0002-9440(10)63108-2]

-
Kim, R.; Kim, E. H.; Ahn, M. J.; Choi, Y. W.; Choi, H. Y.; Kim, S. H.; Byun, J. Y. Arch. Dermatol. Res. 2024, 316, 352.
[https://doi.org/10.1007/s00403-024-03114-w]

-
Lee, M.-J.; Lee, H.-S.; Park, S.-D.; Moon, H.-I.; Park, W.-H. J. Enzyme. Inhib. Med. Chem. 2010, 25, 702–707.
[https://doi.org/10.3109/14756360903524312]

-
Zhang, W.; Hu, Y.; He, J.; Guo, D.; Zhao, J.; Li, P. Front. Pharmacol. 2021, 12, 691995.
[https://doi.org/10.3389/fphar.2021.691995]

-
Lee, Y. J.; Kang, D. G.; Kim, J. S.; Lee, H. S. Vascul. Pharmacol. 2008, 48, 38–46.
[https://doi.org/10.1016/j.vph.2007.11.004]

- Kim, K.-Y.; Oh, T. W.; Ma, J.-Y.; Park, K.-I. Evid. Based Complement. Alternat. Med. 2018, 2018, 9513290.
-
Shin, T.-Y.; Kim, S.-H.; Suk, K.; Ha, J.-H.; Kim, I.; Lee, M.-G.; Jun, C.-D.; Kim, S.-Y.; Lim, J.-P.; Eun, J.-S.; Shin, H.-Y.; Kim, H.-M. Toxicol. Appl. Pharmacol. 2005, 209, 255–262.
[https://doi.org/10.1016/j.taap.2005.04.011]

-
Ellebrecht, C. T.; Maseda, D.; Payne, A. S. J. Invest. Dermatol. 2022, 142, 907–914.
[https://doi.org/10.1016/j.jid.2021.04.040]

-
Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. Cell. Mol. Immunol. 2023, 20, 448–474.
[https://doi.org/10.1038/s41423-023-00992-4]

-
Min, G.-Y.; Kim, E.-Y.; Hong, S.; Kim, J.-H.; Kim, M.; Kim, E. J.; Park, J. H.; Sohn, Y.; Jung, H.-S. Mol. Med. Rep. 2021, 24, 827.
[https://doi.org/10.3892/mmr.2021.12467]

- Gong, G.; Ganesan, K.; Zheng, Y.; Xiao, J.; Dong, T.; Tsim, K. Curr. Med. Chem. 2024, 31, 1–17.