Phytochemical Study on the Aerial Parts of Codonopsis lanceolata
Abstract
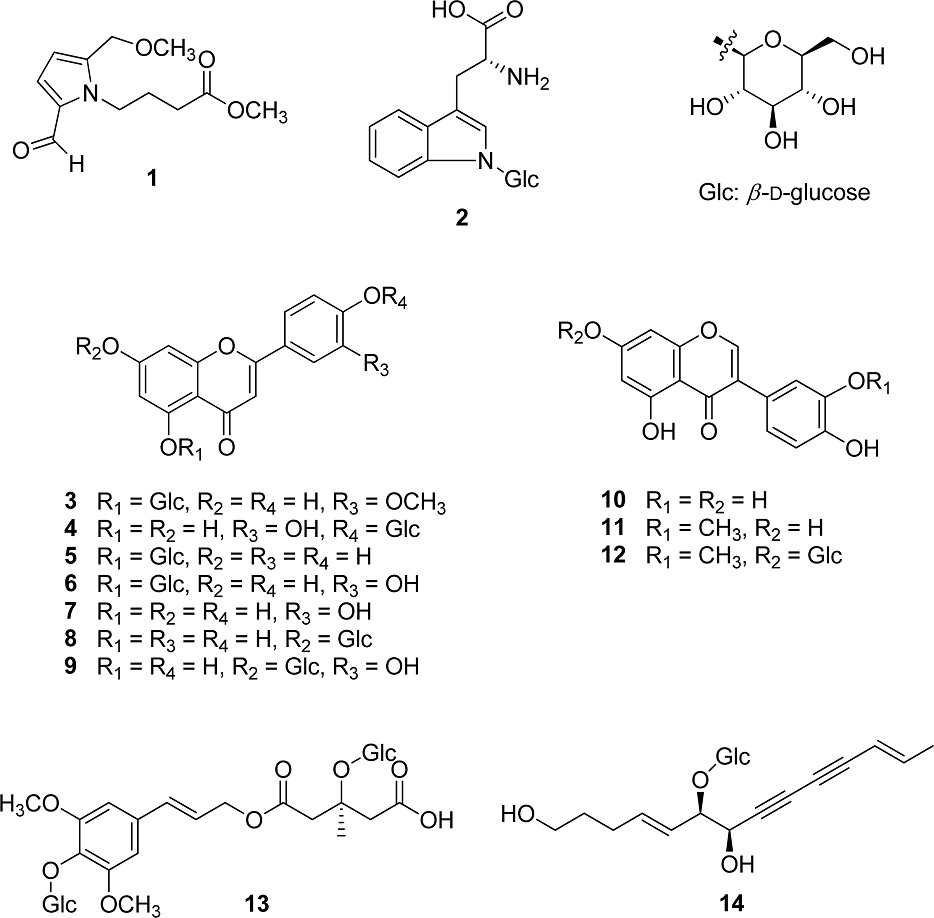
Codonopsis lanceolata, which is predominantly found in Korea and East Asia, has attracted considerable scientific attention due to its long-standing use in traditional medicine. However, despite both its roots and stems being utilized as functional food and medicinal resources, there has been limited phytochemical investigation into its aerial parts. Therefore, the aim of this study is to isolate compounds present in the aerial parts of C. lanceolata. Through repeated chromatography, two alkaloids (1 and 2), seven flavones (3-9), and three isoflavones (10-12) and one type of phenylpropanoid (13) and polyacetylene (14) were isolated from a 70% ethanol extract of C. lanceolata. The structure of each compound was determined through the analysis of spectroscopic data (1H and 13C NMR), and by comparing them with previous research results. Among the isolates, the presence of methyl 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoate (1), chrysoeriol 5-O-β-D-glucopyranoside (3), apigenin-5-O-β-D-glucopyranoside (5), and 3′-O-methylorobol 7-O-β-D-glucopyranoside (12) were reported for the first time in plants belonging to the Campanula family in this study. In addition, the current study marked the isolation of luteolin 4′-O-β-D-glucopyranoside (4) from C. lanceolata for the first time.
Keywords:
Codonopsis lanceolata, Campanulaceae, alkaloid, flavonoidIntroduction
Codonopsis lanceolata Trautv. is a perennial vine belonging to the Campanulaceae family, and it is distributed throughout East Asia, including countries like Korea, Japan, and China. The root of C. lanceolata has traditionally been used as a herbal remedy to treat conditions such as bronchitis, tuberculosis, and disorders related to mental and nervous health.1 In Korea, it has extensive applications not only in traditional herbal medicine but also as a culinary ingredient. According to previous research, due to the significant interest in C. lanceolata roots, extensive studies have been conducted over the past few decades, primarily focusing on the pharmacological effects and various secondary metabolites of C. lanceolata roots.2 The primary components found in C. lanceolata roots encompass phenylpropanoids, saponins, polyphenols, polyacetylenes, tannins, steroids, and alkaloids, all of which have demonstrated notable biological activity. Among these constituents, the representative active compound, lancemaside A, has exhibited antioxidant properties and has been investigated for its potential to mitigate inflammation by inhibiting the NF-κB (Nuclear factor-κB)·IKK (inhibitor of NF-κB kinase) pathway. Additionally, diverse pharmacological effects of lancemaside A have been explored, including stress relief and cell cycle regulation.3-5
As the value of C. lanceolata roots increases due to their various pharmacological effects, there is a gradual increase in research cases utilizing the aerial parts of the plant, such as leaves and stems, in addition to the root.6 However, such research on the aerial parts of C. lanceolata remains relatively limited compared to that on the root. Therefore, the purpose of this study was to isolate compounds present in the aerial parts of C. lanceolata, with the expectation that these compounds may have pharmacological applications and potential for use in functional raw materials.
Experimental
Plant Material – The aerial parts of Codonopsis lanceolata Trautv. were obtained from a domestic farm (Yongmunsan Sandeodeok Farm, Yangpyeong-Gun, Gyeonggi-Do, South Korea), in May 2021. The origin of the herbal material was identified by Prof. Dae Sik Jang and a voucher specimen (COLA4-2021) has been deposited in the Lab. of Natural Product Medicine, College of Pharmacy, Kyung Hee University, Republic of Korea.
General Experimental Procedures – NMR spectra were measured using JEOL 500 MHz (JEOL, Tokyo, Japan). Diaion HP-20 (Mitsubishi, Tokyo, Japan), Sephadex LH-20 (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom), Silica gel (Merck 60A, 70-230 or 230-400 mesh ASTM, Merck, Kenilworth, MA, USA), MCI gel (Supelco, CHP20P) were used for open column chromatography. Medium pressure liquid chromatography (MPLC) was carried out with pre-packed cartridges such as Redi Sep-Silica (12 g, 24 g, 40 g, Teledyne Isco, Lincoln, NE, USA) and Combiflash PF, equipped with Redi Sep-Silica (12 g, 24 g, 40 g, Teledyne Isco) as well as Redi Sep-C18 (13 g, 26 g, 43 g, 130 g, Teledyne Isco) for flash chromatography. Preparative HPLC was conducted using a Waters purification system, which included a 1525 pump and a PDA 1996 detector (Waters, Milford, MA, USA). The separations were performed with a Gemini NX-C18 110A column (250 × 21.2 mm i.d., 5 μm, Phenomenex, Torrance, CA, USA) and a J'sphere ODS-M80 column (250 × 20.0 mm I.D., S-4 μm, 8 nm, YMC, Tokyo, Japan). TLC was performed on silica gel 60 F254 (Merck) and RP-18 F254S (Merck) plate. The spotted TLC plate was visualized by immersing it in 20% (v/v) H2SO4 reagent (Duksan, Seoul, Korea) after solvent development and then heating it at 124oC for 10 minutes. HR-ESI-Orbitrap-MS was obtained by a hybrid Orbitrap mass spectrometer (Q-Exactive, Thermo Fisher Scientific, San Jose, CA, USA).
Extraction and Isolation – The aerial parts of Codonopsis lanceolata (3.6 kg) were extracted twice with 70% ethanol (100 L) over the course of 72 h at room temperature, then the solvent was removed in evaporate at 45℃ to give a 70% EtOH extract (696.89 g).
The 70% ethanol extract (690 g) was chromatographed over Diaion HP-20 (ø 10.4 × 76 cm) as the stationary phase eluting with a MeOH/H2O (0/100 to 100/0, v/v) to give thirteen fractions (Fr. 1~13). Fraction 4 (25.12 g) was divided into seven fractions using Sephadex LH-20 (ø 5.5 × 60.5 cm, MeOH/ H2O = 30/70, v/v). Fr. 4-2 (3.5 g) was subjected to silica gel column chromatography (CC; 230~400 mesh, ø 5.0 × 28.6 cm, CH2Cl2/MeOH/H2O = 60/36/4 to 50/45/5, v/v/v) to yield eight fractions (Fr. 4-2-1~8). Fr. 4-2-7 (2.5 g) was subjected to MCI gel CC (ø 5.0 × 20.7 cm, acetonitrile/H2O = 0/100 to 20/80, v/v) to isolate compound 2 (943.8 mg).
Fraction 6 (7.8 g) was separated into seven fractions (Fr. 6-1~7) using Sephadex LH-20 CC (ø 5.5 × 60.5 cm, MeOH/ H2O = 45/55, v/v). Fr. 6-2 (1.06 g) was subjected to silica gel CC (230~400 mesh, ø 3.5 × 29 cm, CH2Cl2/MeOH/H2O = 100/0/0 to 60/36/4, v/v/v). It was divided into five fractions (Fr. 6-2-1~5), and Fr. 6-2-7 (283.9 mg) was subjected to MCI gel CC (ø 3.0 × 32.0 cm, MeOH/H2O = 30/70 to 40/60, v/v) to separate compound 13 (110.6 mg). Compound 1 (11.0 mg) was separated from Fr. 6-5 (1.06 g) using MPLC with Redi Sep-C18 cartridge (46 g, MeOH/H2O = 35/65 to 55/45, v/v).
Fraction 9 (7.36 g) was separated using silica gel CC (70~230 mesh, ø 4.5 × 37.0 cm, CH2Cl2/MeOH/H2O = 100/0/0 to 40/54/6, v/v/v) into eleven fractions (Fr. 9-1~11). Fr. 9-5 (272.8 mg) was further divided into seven fractions (Fr. 9-5-1~7) using Sephadex LH-20 (ø 2.5 × 52.0 cm, MeOH/H2O = 30/70 to 50/50, v/v). Fr. 9-5-6 (31.8 mg) was subjected to preparative HPLC [(Gemini NX-C18 110A, H2O (0.1% FA, A)/MeOH (0.1% FA, B) = 80:20 to 75:25)] to obtain compound 12 (19.5 mg). Fr. 9-6 (896.3 mg) was fractionated using Sephadex LH-20 (ø 2.5 × 58.2 cm, MeOH/H2O = 35/65, v/v) and produced seven fractions (Fr. 9-6-1~7). Compound 14 (58.2 mg) was isolated from Fr. 9-6-3 (154.6 mg) by MPLC with a Redi Sep-C18 cartridge (46 g, MeOH/H2O = 35/65 to 55/45, v/v). Fr. 9-10 (4.15 g) was fractionated using ODS PR-C18 (ø 4.0 × 26.0 cm, MeOH/H2O = 0/100 to 40/60, v/v) and produced twelve fractions (Fr. 9-10-1~12). Compound 9 (127.3 mg) was isolated from Fr. 9-10-8 (369.7 mg) by recrystallization in MeOH.
Fraction 10 (2.5 g) was separated into ten fractions (Fr. 10-1~10) using Sephadex LH-20 (ø 3.5 × 62.5 cm, MeOH/H2O = 70/30, v/v). Compound 8 (122.4 mg) was separated from Fr. 10-3 (300.0 mg) using MPLC with a Redi Sep-C18 cartridge (46 g, MeOH/H2O = 20/80 to 40/60, v/v). Fr. 10-5 (110.0 mg) was divided into six fractions (Fr. 10- 5-1~6) using MPLC with Redi Sep-C18 cartridge (46 g, MeOH/H2O = 40/60 to 45/55, v/v), among which Fr. 10-5-4 (102.3 mg) was subjected to recrystallization in MeOH to isolate compound 4 (61.0 mg). Fr. 10-8 (180.0 mg) was divided into three fractions (Fr. 10- 8-1~3) using MPLC with a Redi Sep-C18 cartridge (26 g, MeOH/H2O = 20/80 to 50/50, v/v) and Fr. 10-8-2 (158.7 mg) was divided into two fractions (Fr. 10-8-2-1~2) using MPLC with Sep-Silica cartridge (40 g, CH2Cl2/MeOH/H2O = 80/18/2 to 70/27/3, v/v/v). Preparative HPLC (Gemini NX-C18 110A, H2O (0.1% FA, A)/ACN (0.1% FA, B) = 80:20 to 80:20) was conducted on Fr. 10-8-2-2 (142.6 mg) to isolate compounds 3 (4.0 mg), 5 (1.2 mg), and 6 (11.7 mg).
Fraction 12 (9.0 g) was divided into eight fractions (Fr. 12-1~8) by applying Sephadex LH-20 (ø 5.5 × 67.5 cm, MeOH/H2O = 60/40, v/v). Fr. 12-8 (2.0 g) was subjected to silica gel CC (230~400 mesh, ø 4.0 × 23.0 cm, CH2Cl2/MeOH/H2O = 100/0/0 to 50/45/5, v/v/v) to divide into five fractions (Fr. 12-8-1~5). Fr. 12-8-1 (550.9 mg) was recrystallized in MeOH, yielding compound 7 (206.3 mg). Additionally, from the liquid portion (341.6 mg), compound 10 (65.1 mg) was isolated using MPLC with a Redi Sep-C18 cartridge (43 g, MeOH/H2O = 40/60 to 60/40, v/v).
Fraction 13 (64.0 g) was separated into eight fractions (Fr. 13-1~8) using Diaion HP-20 (ø 6.5 × 37.0 cm, acetone/H2O = 50/50 to 100/0, v/v). Fr. 13-4 (3.6 g) was processed with Sephadex LH-20 (ø 5.0 × 53.0 cm, MeOH/ H2O = 80/20, v/v) and divided into nine fractions (Fr. 13-4~19). Fr. 13-4-9 (189.1 mg) was further separated into five fractions (Fr. 13-4-9-1~5) using MCI gel CC (ø 2.0 × 22 cm, MeOH/H2O = 50/50 to 70/30, v/v). Compound 11 (15.5 mg) was isolated through preparative HPLC [(Gemini NX-C18 110A, H2O(A)/ MeOH(B) = 50:50 to 100:00)] from Fr. 13-4-9-4 (33.6 mg).
Methyl 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoate (1) – White powder; HR-ESI-Orbitrap-MS (positive mode) m/z = 240.0549 [M + H]+ (calcd for C12H18NO4, 240.1158); 1H-NMR (CD3OD, 500 MHz) δH : 9.44 (1H, s, 2-CHO), 6.99 (1H, d, J = 4.0 Hz, H-3), 6.29 (1H, d, J = 4.0 Hz, H-4), 4.49 (2H, s, H-6), 4.37 (2H, m, H-1'), 3.66 (3H, s, 4'-OCH3), 3.35 (3H, s, 6-OCH3), 2.36 (2H, t, J = 7.25 Hz, H-3'), 2.01 (2H, q, J = 7.25 Hz, H-2'); 13C-NMR (CD3OD, 125 MHz) δC : 181.3 (2-CHO), 175.3 (C-4'), 141.3 (C-5), 134.1 (C-2), 126.1 (C-3), 113.1 (C-4), 66.5 (C-6), 58.4 (6-OCH3), 52.3 (4'-OCH3), 46.0 (C-1'), 31.8 (C-2'), 27.6 (C-3').7,8
Tryptophan N-glucoside (2) – Yellow powder; 1H NMR (D2O, 500 MHz) δH : 7.72 (1H, d, J = 7.5 Hz, H-8), 7.56 (1H, d, J = 8.0 Hz, H-5), 7.35 (1H, s, H-1), 7.29 (1H, t, J = 7.75 Hz, H-7), 7.2 (1H, t, J = 7.5 Hz, H-6), 5.57 (1H, d, J = 9.0 Hz, H-1'), 4.34 (1H, dd, J = 7.5, 4.5 Hz, H-10), 4.05 (1H, t, J = 9.0 Hz, H-2'), 3.85 (1H, dd, J = 12.5, 2.0 Hz, H-6'), 3.74 (1H, dd, J = 12.0, 5.0 Hz, H-6'), 3.72 (3H, m, H-3', H-4', H-5'), 3.21 (1H, dd, J = 15.0, 4.0 Hz, H-9a), 3.06 (1H, dd, J = 15.0, 7.5 Hz, H-9b); 13C NMR (D2O, 125 MHz) δC : 180.9 (C-11), 136.6 (C-4), 128.6 (C-3), 123.9 (C-1), 122.6 (C-6), 120.4 (C-7), 119.5 (C-8), 112.9 (C-2), 110.2 (C-5), 84.4 (C-1'), 78.2 (C-3'), 76.5 (C-5'), 72.4 (C-2'), 71.6 (C-10), 69.3 (C-4'), 60.5 (C-6'), 30.0 (C-9).9
Chrysoeriol 5-O-β-D-glucopyranoside (3) – Yellow powder; 1H NMR (CD3OD, 500 MHz) δH : 7.48 (1H, dd, J = 8.5, 2.5 Hz, H-6'), 7.45 (1H, d, J = 2.0 Hz, H-2'), 6.92 (1H, d, J = 8.5 Hz, H-5'), 6.82 (1H, d, J = 2.0 Hz, H-8), 6.71 (1H, d, J = 2.0 Hz, H-6), 6.60 (1H, s, H-3), 4.84 (1H, d, J = 8.0 Hz, H-1''), 3.96 (3H, s, OCH3), 3.95 (1H, dd, J = 11.5, 1.5 Hz, H-6''a), 3.77 (1H, dd, J = 12.0, 5.0 Hz, H-6''b), 3.60 (1H, dd, J = 9.0, 7.5 Hz, H-2''), 3.48 (3H, m, H-3'', H-4'', H-5''); 13C NMR (CD3OD, 125 MHz) δC : 180.5 (C-4), 165.1 (C-7), 164.4 (C-2), 160.9 (C-10), 160.3 (C-5), 152.1 (C-9), 149.7 (C-5'), 123.7 (C-1'), 121.7 (C-2'), 117.0 (C-3'), 110.6 (C-6'), 109.6 (C-4'), 107.0 (C-3), 105.2 (C-1''), 105.0 (C-6), 99.5 (C-8), 78.8 (C-5''), 77.5 (C-3''), 74.9 (C-2''), 71.4 (C-4''), 62.7 (C-6''), 56.8 (OCH3).10
Luteolin 4ʹ-O-β-D-glucopyranoside (4) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 12.91 (1H, s, OH-5), 7.52 (1H, dd, J = 8.5, 2.0 Hz, H-6'), 7.50 (1H, d, J = 2.0 Hz, H-2'), 7.24 (1H, d, J = 8.5 Hz, H-5'), 3.83 (1H, s, H-3), 6.50 (1H, d, J = 2.0 Hz, H-8), 6.20 (1H, d, J = 2.0 Hz, H-6), 4.89 (1H, d, J = 7.0 Hz, H-1''), 4.65 (1H, t, J = 5.5 Hz, H-3''), 4.11 (1H, dd, J = 5.5 Hz, H-6''a), 3.73 (1H, ddd, J = 12.0, 5.0, 2.0 Hz, H-5''), 3.48 (1H, dt, J = 12.0, 6.0 Hz, H-6''b), 3.33 (2H, m, H-2'', 4''); 13C-NMR (CD3SOCD3, 125 MHz) δC : 181.8 (C-4), 164.3 (C-7), 163.2 (C-2), 161.5 (C-5), 157.4 (C-9), 148.8 (C-4'), 146.9 (C-3'), 124.7 (C-1'), 118.6 (C-6'), 116.0 (C-5'), 113.6 (C-2'), 104.0 (C-10), 103.8 (C-8), 101.2 (C-1''), 99.0 (C-6), 77.3 (C-5''), 75.9 (C-3''), 73.3 (C-2''), 69.8 (C-4''), 60.71 (C-6'').11
Apigenin 5-O-β-D-glucopyranoside (5) – Yellow powder; 1H NMR (CD3OD, 500 MHz) δH : 7.84 (2H, d, J = 9.0 Hz, H-2', H-6'), 6.93 (2H, d, J = 9.0 Hz, H-3', H-5'), 6.80 (1H, d, J = 2.5 Hz, H-8), 6.67 (1H, d, J = 2.5 Hz, H-6), 6.57 (1H, s, H-3), 4.75 (1H, d, J = 7.5 Hz, H-1''), 3.86 (1H, dd, J = 12.0, 2.0 Hz, H-6''a), 3.68 (1H, dd, J = 12.0, 5.0 Hz, H-6''b), 3.51 (1H, dd, J = 9.0, 7.5 Hz, H-2''), 3.38 (3H, m, H-3'', H-4'', H-5''); 13C-NMR (CD3SOCD3, 125 MHz) δC : 177.4 (C-4), 162.9 (C-2), 161.5 (C-7), 161.1 (C-4'), 158.9 (C-5), 158.6 (C-9), 128.3 (C-2', 6'), 121.4 (C-1'), 116.1 (C-3', 5'), 108.4 (C-10), 105.8 (C-3), 104.5 (C-6), 104.4 (C-1''), 95.8 (C-8), 93.7 (C-2''), 77.6 (C-5''), 75.7 (C-3''), 69.8 (C-4''), 60.9 (C-6'').12
Galuteolin (6) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 7.37 (2H, m, H-2', H-6'), 6.88 (1H, d, J = 8.0 Hz, H-5'), 6.79 (1H, d, J = 2.0 Hz, H-8), 6.70 (1H, d, J = 2.5 Hz, H-6), 6.55 (1H, s, H-3), 4.71 (1H, d, J = 7.5 Hz, H-1''), 3.75 (1H, dd, J = 12.0, 2.5 Hz, H-6''a), 3.54 (1H, dd, J = 12.0, 5.5 Hz, H-6''b), 3.35 (2H, m, H-2'', H-5''), 3.28 (1H, t, J = 8.5 Hz, H-3''), 3.19 (1H, m, H-4''); 13C NMR (CD3SOCD3, 125 MHz) δC : 177.0 (C-4), 162.9 (C-2), 161.4 (C-7), 158.7 (C-5), 158.4 (C-9), 149.3 (C-4'), 145.8 (C-3'), 121.5 (C-1'), 118.6 (C-6'), 116.0 (C-5'), 113.1 (C-2'), 108.2 (C-10), 105.7 (C-3), 104.6 (C-1''), 104.5 (C-6), 98.2 (C-8), 77.6 (C-5''), 75.6 (C-3''), 73.7 (C-2''), 70.0 (C-4''), 60.9 (C-6'').13
Luteolin (7) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 7.42 (1H, dd, J = 2.0, 8.0 Hz, H-6'), 7.39 (1H, d, J = 2.0 Hz, H-2'), 6.89 (1H, d, J = 8.0 Hz, H-5'), 6.67 (1H, s, H-3), 6.44 (1H, d, J = 2.0 Hz, H-8), 6.18 (1H, d, J = 2.0 Hz, H-6).14
Apigenin 7-O-β-D-glucopyranoside (8) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 7.96 (2H, d, J = 9.0 Hz, H-2', H-6'), 6.94 (2H, d, J = 9.0 Hz, H-3', H-5'), 6.88 (1H, s, H-3), 6.83 (1H, d, J = 2.0 Hz, H-8), 6.44 (1H, d, J = 2.0 Hz, H-6), 5.07 (1H, d, J = 7.5 Hz, H-1''), 3.67 (1H, d, J = 11.0 Hz, H-6''a), 3.41 (2H, m), 3.20 (3H, m).15
Luteolin 7-O-β-D-glucopyranoside (9) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 13.0 (1H, s, OH-5), 7.45 (1H, dd, J = 8.5, 2.0 Hz, H-2'), 7.42 (1H, d, J = 2.0 Hz, H-6'), 6.90 (1H, d, J = 8.5 Hz, H-3'), 6.79 (1H, d, J = 2.0 Hz, H-8), 6.76 (1H, s, H-3), 6.44 (1H, d, J = 2.0 Hz, H-6), 5.08 (1H, d, J = 7.5 Hz, H-1''), 3.71 (1H, d, J = 10.5 Hz, H-6''a), 3.47 (2H, m), 3.28 (2H, m), 3.18 (1H, d, J = 9.0 Hz, H-2'').16
Orobol (10) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 13.0 (1H, s, OH-5), 8.29 (1H, s, H-2), 6.99 (1H, d, J = 2.0 Hz, H-2'), 6.78 (1H, m, H-5', H-6'), 3.67 (1H, d, J = 2.0 Hz, H-8), 6.21 (1H, d, J = 2.0 Hz, H-6); 13C NMR (CD3SOCD3, 125 MHz) δC : 180.3 (C-4), 164.4 (C-10), 162.0 (C-5), 157.6 (C-9), 154.0 (C-2), 145.6 (C-4'), 144.9 (C-3'), 122.4 (C-3), 121.6 (C-1'), 120.0 (C-6'), 116.6 (C-2'), 115.4 (C-5'), 104.5 (C-7), 99.0 (C-6), 93.7 (C-8).17
3′-O-Methylorobol (11) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 12.97 (1H, s, OH-5), 8.34 (1H, s, H-2), 7.13 (1H, d, J = 2.0 Hz, H-2'), 6.98 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 6.82 (1H, d, J = 8.0 Hz, H-5'), 6.38 (1H, d, J = 2.0 Hz, H-8), 6.22 (1H, d, J = 2.0 Hz, H-6), 3.79 (1H, s, OCH3).18
3′-O-Methylorobol 7-O-β-D-glucopyranoside (12) – Yellow powder; 1H NMR (CD3SOCD3, 500 MHz) δH : 12.96 (1H, s, OH-5), 8.47 (1H, s, H-2), 7.16 (1H, d, J = 2.0 Hz, H-2'), 7.01 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 6.84 (1H, d, J = 8.0 Hz, H-5'), 6.73 (1H, d, J = 2.0 Hz, H-8), 6.47 (1H, d, J = 2.0 Hz, H-6), 5.07 (1H, d, J = 7.5 Hz, H-1''), 3.80 (1H, s, OCH3), 3.70 (1H, d, J = 10.0 Hz, H-6''a), 3.46 (3H, m), 3.28 (2H, m); 13C NMR (CD3SOCD3, 125 MHz) δC : 180.5 (C-4), 163.0 (C-10), 161.7 (C-5), 157.2 (C-9), 154.9 (C-2), 147.3 (C-4'), 146.8 (C-3'), 122.6 (C-3), 121.7 (C-1'), 121.4 (C-6'), 115.3 (C-2'), 113.2 (C-5'), 106.1 (C-1''), 99.8 (C-6), 99.6 (C-8), 94.5 (C-7), 77.2 (C-5''), 76.4 (C-3''), 73.1 (C-2''), 69.6 (C-4''), 60.6 (C-6''), 55.7 (OCH3).19
Tangshenoside I (13) – Yellow powder; 1H NMR (D2O, 500 MHz) δH : 6.62 (2H, s, H-2, H-6), 6.50 (1H, d, J = 16.0 Hz, H-γ), 6.22 (1H, dt, J = 15.5, 6.5 Hz, H-β), 5.0 (1H, d, J = 7.0 Hz, Glc H-1') 4.76 (2H, d, J = 6.0 Hz, H-α), 4.70 (1H, d, J = 8.0 Hz, Glc H-1), 3.85 (6H, s, OCH3×2), 3.79 (2H, m), 3.69 (1H, dd, J = 12.5, 5.5 Hz, Glc H-6b), 3.63 (1H, dd, J = 12.5, 5.5 Hz, Glc H-6'b), 3.54 (2H, m), 3.47 (2H, m), 3.39 (1H, ddd, J = 10.0, 5.5, 2.5, Glc H-5), 3.32 (2H, m), 3.24 (1H, dd, J = 9.5, 8.0 Hz, Glc H-4), 2.92 (2H, s, H-2'a, H-2'b), 2.65 (2H, q, J = 14.0 Hz, H-4a', H-4b'), 1.49 (3H, s, H-6'); 13C-NMR (D2O, 125 MHz) δC : 176.7 (C-5'), 173.3 (C-1'), 153.3 (C-3, C-5), 134.4 (C-γ), 134.4 (C-4), 134.2 (C-1), 124.3 (C-β), 105.1 (C-2, C-6), 103.8 (Glc, C-1), 97.2 (Glc, C-1'), 78.2 (C-3'), 77.0 (Glc, C-3), 76.6 (Glc, C-3'), 76.6 (Glc, C-5), 76.5 (Glc, C-5'), 74.5 (Glc, C-2), 74.0 (Glc, C-2'), 70.3 (Glc, C-4), 70.0 (Glc, C-4'), 66.3 (C-α), 61.5 (Glc, C-6), 61.2 (Glc, C-6'), 57.0 (OCH3×2), 47.4 (C-4'), 44.3 (C-2'), 24.8 (C-6').20
Lobetyolin (14) – Brown powder; 1H NMR (CD3COCD3, 500 MHz) δH : 6.31 (1H, dq, J = 16.0, 7.0 Hz, H-2), 5.89 (1H, dt, J = 15.5, 7.0 Hz, H-11), 5.58 (1H, dd, J = 16.0, 2.0 Hz, H-3), 5.43 (1H, ddd, J = 15.5, 8.0, 1.5 Hz, H-10), 4.41 (1H, d, J = 7.0 Hz, H-8), 4.35 (1H, d, J = 8.0 Hz, H-1'), 4.22 (1H, t, J = 7.0 Hz, H-9), 3.80 (1H, dd, J = 12.0, 2.5 Hz, H-6'b), 3.63 (1H, dd, J = 12.0, 6.0 Hz, H-6'a), 3.53 (2H, t, J = 6.5 Hz, H-14), 3.35 (1H, t, J = 8.0 Hz, H-4'), 3.24 (3H, m, H-2', H-3', H-5'), 2.13 (2H, m, H-12), 1.76 (3H, dd, J = 7.0, 2.0 Hz, H-1), 1.58 (2H, p, J = 7.5 Hz, H-13); 13C-NMR (CD3COCD3, 125 MHz) δC : 145.3 (C-2), 138.9 (C-11), 126.5 (C-10), 110.4 (C-3), 100.7 (C-1'), 81.8 (C-4), 81.1 (C-9), 78.1 (C-5), 78.0 (C-3'), 78.0 (C-5'), 74.8 (C-2'), 72.5 (C-6), 71.6 (C-4'), 71.2 (C-7), 66.6 (C-8), 62.7 (C-6'), 62.2 (C-14), 32.9 (C-13), 29.8 (C-12), 18.9 (C-1).21
Results and Discussion
Through repeated chromatography, a total of 14 compounds were isolated from a 70% ethanol extract from the aerial parts of C. lanceolata. The chemical structures of the isolated compounds (1–14) were identified based on spectroscopic data (NMR and LC-MS) and comparison with previous research. They were determined as methyl 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoate (1),7,8 tryptophan N-glucoside (2),9 chrysoeriol 5-O-β-D-glucopyranoside (3),10 luteolin 4′-O-β-D-glucopyranoside (4),11 apigenin 5-O-β-D-glucopyranoside (5),12 galuteolin (6),13 luteolin (7),14 apigenin 7-O-β-D-glucopyranoside (8),15 luteolin 7-O-β-D-glucopyranoside (9),16 orobol (10),17 3′-O-methylorobol (11),18 3′-O-methylorobol 7-O-β-D-glucopyranoside (12),19 tangshenoside I (13),20 and lobetyolin (14).21
In this study, methyl 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoate (1), chrysoeriol 5-O-β-D-glucopyranoside (3), apigenin 5-O-β-D-glucopyranoside (5), and 3′-O-methylorobol 7-O-β-D-glucopyranoside (12) were isolated from Campanulaceae family for the first time, while luteolin 4′-O-β-D-glucopyranoside (4) was isolated from C. lanceolata for the first time.
Luteolin 4′-O-β-D-glucopyranoside (4), a compound first isolated from C. lanceolata, has notable ability to inhibit the activity of TNF-α, a tumor necrosis factor that causes inflammatory reactions and immune diseases.22 Beyond its direct suppression of TNF-α, luteolin 4′-O-β-D-glucopyranoside (4) shows promise in suppressing other inflammatory pathways, including potent inhibition of NF-κB which is a transcription factor involved in managing inflammation and immune-related disorders.23 Moreover, 4 has shown potent anti-inflammatory effects by inhibiting IL-5, a chemotactic factor crucial in allergic inflammation, with notable efficacy.24 Meanwhile, previous study showed that luteolin 4′-O-β-D-glucopyranoside (4) alleviated symptoms of hyperuricemia and gouty arthritis, by decreasing the levels of IL-1β and TNF-α.25 Therefore, potential synergistic effects of luteolin 4′-O-β-D-glucoside (4) in downregulating both pro-inflammatory cytokines and critical cellular signaling pathways underline its promise as an agent for both cancer prevention and therapy.
While these findings highlight the medicinal promise of C. lanceolata, other compounds such as methyl 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoate (1), chrysoeriol 5-O-β-D-glucopyranoside (3), and 3′-O-methylorobol 7-O-β-D-glucopyranoside (12) remain less characterized in terms of their pharmacological actions. This research broadens our knowledge of the phytochemicals from the aerial parts of C. lanceolata, yet it also suggests that additional research on the aerial parts of C. lanceolata is needed.
Acknowledgments
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT), Republic of Korea (Grant no. NRF-2019R1A2C1083945). The authors would like to thank Nam Sang Cho, Yongmunsan Sandeodeok Farm owner, for providing the sample materials.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Sathiyamoorthy, S.; In, J. G.; Lee, O. R.; Lee, B. S.; Devi, S. R.; Yang, D. C. Mol. Biol. Rep. 2011, 38, 3541-3549.
[https://doi.org/10.1007/s11033-010-0464-9]

-
Hossen, M. J.; Kim, M. Y.; Kim, J. H.; Cho, J. Y. Phytother. Res. 2016, 30, 347-356.
[https://doi.org/10.1002/ptr.5553]

-
He, X.; Yoon, W. B.; Park, S. J.; Park, D. S.; Ahn, J. Food Sci. Biotechnol. 2011, 20, 499-505.
[https://doi.org/10.1007/s10068-011-0069-7]

-
Du, Y. E.; Lee, J. S.; Kim, H. M.; Ahn, J. H.; Jung, I. H.; Ryu, J. H.; Choi, J. H.; Jang, D. S. Arch. Pharm. Res. 2018, 41, 1082–1091.
[https://doi.org/10.1007/s12272-018-1080-9]

-
Xie, Q.; Hu, X.; Zhao, X.; Xiang, Z.; Chen, Q.; Xie, Z.; Wang, H; Zhao, Y; Cheng, X.; Wang, C. J. Ethnopharmacol. 2024, 319, 117106.
[https://doi.org/10.1016/j.jep.2023.117106]

-
Kim, J. Y.; Hwang, B. S.; Kwon, S. H.; Jang, M.; Kim, G. C.; Kang, H. J.; Hwang, I. G. J. Korean Soc. Food Sci. Nutr. 2021, 50, 10-15.
[https://doi.org/10.3746/jkfn.2021.50.1.10]

-
Chin, Y. W.; Lim, S. W.; Kim, S. H.; Shin, D. Y.; Suh, Y. G.; Kim, Y. B.; Kim, Y. C.; Kim, J. W. Bioorg. Med. Chem. Lett. 2003, 13, 79-81.
[https://doi.org/10.1016/S0960-894X(02)00846-6]

-
Liu, W. Y.; Zhang, W. D.; Chen, H. S.; Gu, Z. B.; Li, T. Z.; Yun-Zhou. J. Asian Nat. Prod. Res. 2003, 5, 159-163.
[https://doi.org/10.1080/1028602031000066861]

-
Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Mártonfi, P.; Maggi, F. Pharm. Biol. 2017, 55, 1162-1170.
[https://doi.org/10.1080/13880209.2017.1291689]

-
Lee, M. H.; Son, Y. K.; Han, Y. N. Arch. Pharm. Res. 2002, 25, 842-850.
[https://doi.org/10.1007/BF02977002]

-
Veit, M.; Geiger, H.; Czygan, F. C.; Markham, K. R. Phytochemistry 1990, 29, 2555-2560.
[https://doi.org/10.1016/0031-9422(90)85187-K]

- Lin, J. H.; Lin, Y. T.; Huang, Y. J.; Wen, K. C.; Chen, R. M.; Ueng, T. H.; Liao, C. H. J. Food Drug Anal. 2001, 9, 6-11.
-
Hsu, H. F.; Houng, J. Y.; Chang, C. L.; Wu, C. C.; Chang, F. R.; Wu, Y. C. J. Agric. Food Chem. 2005, 53, 6117-6125.
[https://doi.org/10.1021/jf050463u]

- Piao, M. S; Kim, M. R.; Lee, D. G.; Pack, Y. K.; Hahm, K. S.; Moon, Y. H.; Woo, E. R. Arch. Pharm. Res. 2003, 26, 453-457.
-
Veit, M.; Geiger, H.; Czygan, F. C.; Markham, K. R. Phytochem. 1990, 29, 2555-2560.
[https://doi.org/10.1016/0031-9422(90)85187-K]

-
VanWagenen, B. C.; Huddleston, J.; Cardellina, J. H. J. Nat. Prod. 1988, 51, 136-141.
[https://doi.org/10.1021/np50055a021]

-
Van Heerden, F. R.; Brandt, E. V.; Roux, D. G. J. Chem. Soc. Perkin Trans. 1. 1980, 2463-2469.
[https://doi.org/10.1039/p19800002463]

-
Hanawa, F.; Tahara, S.; Mizutani, J. Phytochemistry 1991, 30, 157-163.
[https://doi.org/10.1016/0031-9422(91)84117-B]

- Viscardi, P.; Reynaud, J.; Raynaud, J. Pharmazie 1984, 39, 781.
-
Mizutani, K.; Yuda, M.; Tanaka, O.; Saruwatari, Y. I.; Jia, M. R.; Ling, Y. K.; Pu, X. F. Chem. Pharm. Bull. 1988, 36, 2726-2729.
[https://doi.org/10.1248/cpb.36.2726]

-
Ishimaru, K.; Yonemitsu, H.; Shimomura, K. Phytochemistry 1991, 30, 2255-2257.
[https://doi.org/10.1016/0031-9422(91)83624-T]

- Lee, Y. H.; Lim, Y. H.; Lee, S. H.; Yong, Y. J.; Woo, Y. K.; Shin, S. Y. Korea Patent 10-2010-0134966, 2009.
-
De Marino, S.; Festa, C.; Zollo, F.; Nini, A.; Antenucci, L.; Raimo, G.; Iorizzi, M. Anticancer Agents Med. Chem. 2014, 14, 1376-1385.
[https://doi.org/10.2174/1871520614666140804153936]

-
Lin, Y.; Liu, P. G.; Liang, W. Q.; Hu, Y. J.; Xu, P.; Zhou, J.; Pu, J. B.; Zhang, H. J. Phytomedicine 2018, 41, 54-61.
[https://doi.org/10.1016/j.phymed.2018.02.002]

-
Park, K. Y.; Lee, S. H.; Min, B. K.; Lee, K. S.; Choi, J. S.; Chung, S. R.; Min, K. R.; Kim, Y. S. Planta Med. 1999. 65, 457-459.
[https://doi.org/10.1055/s-2006-960812]