Systematic Evaluation and Meta-Analysis of the Effect of Gynostemma pentaphyllum on Clinical Indexes of Hyperlipidemia
Abstract
The purpose of this study was to explore the clinical efficacy and safety of Gynostemma pentaphyllum (G. pentaphyllum) in the treatment of hyperlipidemia, and to provide systematic evaluation basis for clinical application. CNKI, Wanfang Data, VIP, Web of science, PubMed, Embase and Cochrane Library were searched for randomized controlled trials (RCTs) about G. pentaphyllum in the treatment of hyperlipidemia. Review Manager 5.4 were used for statistical analysis. Through reading topics, abstracts, and full texts, 27 papers with 2311 cases involved that met the inclusion and exclusion criteria were finally included for the analysis. In terms of curative effect, the effect of G. pentaphyllum alone in increasing high density lipoprotein (HDL) index was better than that of conventional treatment, and the effect of reducing total cholesterol (TC), triglyceride (TG) and low density lipoprotein (LDL) was similar to that of conventional treatment. There was a synergistic effect between G. pentaphyllum and conventional drugs, and the combination of G. pentaphyllum and conventional drugs was superior to conventional treatment in reducing TG and increasing HDL. G. pentaphyllum can also decrease the levels of serum glutamic pyruvic transaminase and glutamic oxaloacetic transaminase in the treatment of hyperlipidemia, indicating a certain protective function of the liver. In terms of safety, there were fewer cases of adverse reactions in the G. pentaphyllum treatment group, and the adverse reaction events reported in the literature was mild. According to the results of meta-analysis, G. pentaphyllum was effective in the treatment of hyperlipidemia, and it has the potential to be combined with traditional drugs, has a certain liver protection function, and was superior to traditional drugs in the treatment of hyperlipidemia.
Keywords:
Gynostemma pentaphyllum, Hyperlipidemia, Meta-analysis, Clinical evaluationIntroduction
Hyperlipidemia was a significant risk factor for cardiovascular disease, associated with serious consequences such as fatty liver, atherosclerosis, and sudden death.1-4 It primarily refers to elevated levels of total cholesterol (TC), triglycerides (TG), or low-density lipoprotein (LDL), or reduced levels of high-density lipoprotein (HDL) in the serum. This condition commonly occurs among middle-aged and elderly individuals and has shown an increasing incidence due to improved living standards and changes in lifestyle habits. Hyperlipidemia was a global concern, ranking highest in both age-standardized death rate and age-standardized disability-adjusted life years.5 Various therapeutic options were available for clinical management, including statins, ezetimibe, bempedoic acid, inclisiran, and volanesorsen.6 While these interventions have a defined therapeutic effect, they were often associated with adverse effects, such as statin-associated muscle symptoms (SAMS), connective tissue disorders, and gastrointestinal disturbances.7-9 Despite the utilization of these treatments, our understanding of the effectiveness and safety in the management of hyperlipidemia remains limited. The primary challenge in hyperlipidemia treatment lies in identifying effective methods to implement the currently available intervention measures.
Gynostemma pentaphyllum (Thunb.) Makino, a member of the Cucurbitaceae family, is distributed in East Asia, Tropical Asia, New Guinea, and the Himalayas. It has been primarily utilized for the treatment of hyperlipidemia, atherosclerosis, myocardial infarction, coronary heart disease, and cardiovascular disease.10-15 Numerous studies have reported the use of G. pentaphyllum in combating hyperlipidemia. However, the clinical research on G. pentaphyllum remains limited with a small sample size, potentially impairing an accurate assessment of its efficacy in hyperlipidemia treatment. Consequently, this study conducted a systematic evaluation and meta-analysis of clinical articles pertaining to G. pentaphyllum’s treatment of hyperlipidemia. The aim of this study was to provide an accurate reflection of the clinical efficacy of G. pentaphyllum in treating hyperlipidemia, offering guidance for its clinical application and future research.
Experimental
Database and search strategies – In this study, seven databases were selected for all the randomized, controlled clinical trials concerning G. pentaphyllum for the treatment of hyperlipidemia, fatty liver, hyperlipidemia with diabetes and other related topics. We perform a systematic literature search in Web of Science, PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang data, and VIP database. The deadline for searching all the above electronic databases was March 14, 2023. The keywords used in the search included G. pentaphyllum, hyperlipidemia, cholesterol, low-density lipoprotein, high-density lipoprotein, and random. A retrospective search of the references cited in the literature was performed to minimize the likelihood of data omission. This process involved reviewing the bibliography of relevant articles to identify additional sources that provide relevant information and contribute to a more comprehensive analysis.
Inclusion and exclusion criteria – The inclusion criteria for this study are as follows: (1) Subjects: The patient was diagnosed with hyperlipidemia according to the Chinese guidelines for the Management of Blood Lipid (2023), with TC ≥ 6.2 mmol/L, TG ≥ 2.3 mmol/L, LDL ≥ 4.1 mmol/L, HDL ≤ 1.0 mmol/L in fasting venous plasma. Concurrent comorbidities such as diabetes and coronary heart disease were also considered eligible. (2) Intervention: The treatment group received G. pentaphyllum or G. pentaphyllum total saponins, while the control group did not receive any treatment, received placebo treatment, or received lipid-lowering drug treatment, which may include statins among other medications. The duration of treatment was a minimum of 4 weeks. (3) Outcome indicators: The primary outcome indicators assessed were TC, TG, LDL, and HDL, while secondary indicators included alanine aminotransferase (ALT), aspartate aminotransferase (AST), and adverse reactions. (4) Types of studies: All included studies are randomized controlled trials (RCT). The exclusion criteria were as follows: (1) Repeatedly published literature. (2) It was impossible to obtain the full-text literature. (3) The literature of inconsistent intervention measures. (4) The literature with inconsistent outcome index. (5) The literature in which the case index was not consistent. (6) Retrospective study. (7) Non-G. pentaphyllum experimental group.
Literature screening – The retrieved data was organized and managed using NoteExpress software. Duplicate documents were identified and removed from the dataset to ensure data integrity and avoid redundancy. A preliminary reading of the literature was conducted, encompassing the examination of titles and abstracts. This initial screening process aimed to eliminate literature that clearly did not align with the topic under investigation. Subsequently, a more comprehensive screening of the literature was performed by reading the full texts. This stage involved applying predefined criteria for inclusion and exclusion of literature to select relevant studies that could be incorporated into the research. The process of literature screening was carried out independently by two researchers. The screening results were then compared and any divergent literature was thoroughly discussed to reach a consensus. In case of disagreement, a third experienced researcher was consulted to provide insight and expertise to facilitate resolution.
Data extraction – During the data extraction process, two researchers independently extracted the data based on a predefined data extraction table. Each researcher performed the data extraction individually and subsequently cross-checked their findings. If any discrepancies arose during data extraction, they were discussed and resolved through consensus. Alternatively, an experienced third researcher was consulted as needed to contribute to the resolution of any discrepancies. Once an agreement was reached, the final data extraction results were generated. The following information was extracted from the included studies: data extraction table encompassed the following contents: (1) Basic information of the study: including the research topic, first author, and research timeframe. (2) Methodological information: including details of the study design and information required for assessing bias risk. (3) Subject characteristics: encompassing sex and proportion, age, disease name, type of hyperlipidemia, and any complications. (4) Grouping: comprising sample size, intervention measures, and treatment duration for both the test group and the control group. (5) Outcome indicators: outlining the specific measures used to evaluate the outcomes of interest. These elements formed the key information extracted from each study to support the analysis and synthesis of data in the research project.
Offset risk assessment – The evaluation process involved independent assessments by two researchers, followed by cross-checking and discussion at the analysis stage or consultation with an experienced third party. The bias risk assessment tool integrated within Review Manager (RevMan) 5.4 software was utilized for this purpose. The evaluation encompassed the following aspects: (1) Random allocation method. (2) Concealed allocation scheme. (3) Blinding of study subjects and implementers of treatment regimens. (4) Blinding of outcome measurement. (5) Data integrity. (6) Selective reporting of research results. (7) Other potential sources of bias. Each literature included in the study was assessed across these seven aspects, using predefined assessment criteria. The assessment criteria were categorized into three levels: ‘high risk’, ‘low risk’, and ‘unclear’. The evaluation process aimed to determine the risk of bias associated with each study, ensuring a critical appraisal of the evidence to inform the overall analysis and interpretation of the findings.
Data synthesis and statistical analysis – RevMan 5.4 software was utilized for conducting the meta-analysis, employing the inverse-variance (IV) algorithm as the statistical method. For studies with continuous variables, the standardized mean difference (SMD) was employed as the statistical measure of effect size. On the other hand, when the research data involved binary variables, the effect statistical method used was the relative risk (RR). The analysis was performed with a confidence interval (CI) set at 95%. To assess the heterogeneity among studies, the magnitude of I2 was considered. When I2 was below 50%, it indicated a low level of heterogeneity among the studies, and the fixed-effect model (FEM) was employed to analyze the effect. The effect size of each study was proportional to the sample size of the study. Conversely, when I2 exceeded 50%, it suggested a substantial level of heterogeneity, necessitating the use of the random-effects model (REM) for analysis. If I2 exceeded 75%, it indicated a very high degree of heterogeneity. In addition to utilizing the random-effects model during the analysis, subgroup analysis was conducted to identify the potential causes of the observed heterogeneity. To ensure the stability of the results, a sensitivity analysis was performed, evaluating the robustness and consistency of the findings. This analysis aimed to assess the impact of individual studies on the overall results and determine the reliability of the conclusions drawn from the meta-analysis.
Results and Discussion
A comprehensive search was conducted across multiple databases, including PubMed, Cochrane Library, EMBASE, Web of Science, CNKI, Wanfang Database, and VIP Database. Among the initial pool of 651 articles, a meticulous assessment of titles and abstracts led to the selection of 121 studies for further evaluation. Out of these, 94 studies were subsequently excluded based on specific criteria: 2 articles were duplicates, 1 involved retrospective analysis, another was a review, 71 studies did not comply with intervention measures, 4 studies lacked compliance with case indicators, 6 studies exhibited inconsistent outcome indicators, 8 studies had incomplete data, and 1 article was excluded due to unavailability of the full text. As a result, a final selection of 27 studies, encompassing 2311 subjects, was included for the subsequent analysis (Fig. 1).
The characteristics of the literature included in the study were shown in Table 1.
The random grouping was mentioned in all the included literature; however, only six studies provided specific details regarding the methods of randomization. These six studies were thus classified as ‘low risk’ in terms of randomization, while the remaining studies were assessed as ‘Some concerns’. Regarding the completeness of reporting, studies that reported a comprehensive set of outcome indicators, including TC, TG, HDL, and LDL, were categorized as ‘low risk’. On the other hand, studies that did not provide a complete set of these indicators were classified as ‘Some concerns’. None of the included studies mentioned specific methods for allocation concealment. However, since all articles mentioned ‘randomized grouping,’ the analysis assessment of allocation concealment in this study is categorized as ‘Some concerns’. The included studies did not mention whether blinding was utilized. Therefore, this study categorizes the included studies as having a ‘high risk’ in terms of blinding. Based on the results of the funnel plot presented later in the text, this study suggests that one of the included studies may have a potential for Selective reporting. The results are shown in Table 2.
Among the main outcome indicators, TC levels were assessed in 23 cases, TG levels in 27 cases, LDL levels in 21 cases, and HDL levels in 23 cases. All assessments pertain to the comparison between the G. pentaphyllum experimental group and the control group (Table 3). The findings revealed that the G. pentaphyllum experimental group exhibited a better reduction in TC levels compared to the conventional treatment group. However, the observed differences between the two groups were not statistically significant (SMDTC = –0.36, 95% CI [–1.15, 0.44], p = 0.38 > 0.05). Regarding the impact on TG levels, the G. pentaphyllum experimental group demonstrated a significantly superior ability to decrease TG levels in comparison to the conventional treatment group (SMDTG = –1.48, 95% CI [–2.13, –0.83], p < 0.01). In terms of the effect on LDL levels, the G. pentaphyllum experimental group exhibited a significantly better reduction in LDL levels compared to the control group (SMDLDL = –1.37, 95% CI [–2.38, –0.36], p < 0.01). Furthermore, regarding the influence on HDL levels, the G. pentaphyllum experimental group demonstrated a significantly stronger ability to increase HDL levels compared to the control group (SMDHDL = 1.43, 95% CI [0.97, 1.89], p < 0.01).
Among the secondary outcome indicators, 5 cases of ALT and 6 cases of AST were included in the study, comparing the G. pentaphyllum experimental group with the conventional treatment control group (Table 4). Regarding the impact on ALT index, the G. pentaphyllum group exhibited a significantly better ability to reduce ALT index compared to the conventional treatment group (SMDALT = –1.83, 95% CI [–3.16, –0.50], p < 0.01). Similarly, concerning the effect on AST index, the G. pentaphyllum group showed a significantly better ability to reduce AST index in comparison to the conventional treatment group (SMDAST = –1.81, 95% CI [–3.40, –0.23], p < 0.05).
Significant heterogeneity was observed among the various studies for each index, as evident by the statistical significance (p < 0.01) and high inconsistency (I2 = 98%). Due to the limited number of included studies, it was not feasible to perform subgroup analysis for ALT and AST indexes. Consequently, further discussion pertaining to AST and ALT indexes was omitted, focusing solely on the main indexes: TC, TG, LDL, and HDL. The observed heterogeneity was primarily attributed to the differences in single drug use and combined drug use among the studies. To address this heterogeneity, subgroup analysis and sensitivity analysis were conducted based on the different interventions employed in each study to discern patterns and potential sources of inconsistency.
Following the subgroup analysis of the included literature in this study, substantial heterogeneity persisted within each subgroup. Additionally, articles exhibiting lower levels of heterogeneity were identified using forest plots for comparative purposes. These articles with minimal heterogeneity demonstrated variations in factors such as intervention, age, gender composition, and duration of treatment. Conversely, articles sharing similar influencing factors displayed a high degree of heterogeneity. Consequently, it can be inferred that this heterogeneity may be attributed to unreported factors present in these literature sources. The analysis results from each subgroup revealed that the use of G. pentaphyllum alone as the experimental group yielded superior outcomes compared to the control group in terms of increasing the HDL index. The effectiveness in reducing TC, TG, and LDL indexes was comparable to that of routine treatment. The combination group demonstrated a more favorable effect, surpassing the control group in reducing TG levels and increasing HDL levels. Detailed results of the subgroup analysis can be found in Table 5. The findings of the sensitivity analysis were presented in Table 6.
In 12 studies, G. pentaphyllum alone was used as the experimental group and in 11 studies, G. pentaphyllum was used in combination with lipid-lowering drugs. The heterogeneity test showed that there was great heterogeneity between the G. pentaphyllum group (Chi2 = 392.59, p < 0.01, I2 = 97%). and the combination group (Chi2 = 655.95, p < 0.01, I2= 98%). The random effect model was used for analysis. The results showed that the TC reduction ability of G. pentaphyllum alone was better than that of the control group, but there was no statistical difference between the two groups (SMDalone = –0.42, 95% CI [–1.33, 0.50], p = 0.37 > 0.05). The ability of reducing TC in the combination group was also higher than that in the control group, but there was also no statistical difference (SMDcombination = –0.19, 95% CI [–1.57, 1.19], p = 0.78 > 0.05). As substantial heterogeneity persisted even after subgroup analysis, a sensitivity analysis was conducted by excluding the studies with the highest item heterogeneity within each subgroup. After excluding these two studies, both the subgroup trends and overall results remained consistent, indicating good stability in the data analysis.
In 14 studies, G. pentaphyllum alone was used as the experimental group and 13 studies used combined drugs as the experimental group. After heterogeneity test, there was great heterogeneity between G. pentaphyllum group (Chi2 = 335.29, p < 0.01, I2 = 96%) and combination group (Chi2 = 634.42, p < 0.01, I2 = 98%). The random effect model was used for analysis. The results showed that the TG reduction ability of G. pentaphyllum alone was stronger than that of the control group, but there was no statistical difference between the two groups (SMDalone = –0.36, 95% CI [–1.02, 0.31], p = 0.29 > 0.05). The ability of reducing TG in the combination group was stronger than that in the control group, and the result was statistically different (SMDcombination = –2.83, 95% CI [–3.99, –1.67], p < 0.01). Given the consistently high level of heterogeneity observed following subgroup analysis, the present study conducted a sensitivity analysis for each subgroup. The sensitivity analysis involved excluding the top two studies with the highest item heterogeneity within each subgroup and subsequently observing whether there were any changes in the statistical results. As a result of excluding these two studies, the trends observed in the subgroups and overall results underwent changes (SMDalone = –0.60, 95% CI [–1.10, –0.10], p < 0.05). This indicated that the conclusion was unstable, possibly due to the inclusion of relatively low-quality literature. Consequently, a detailed discussion regarding the effect of G. pentaphyllum alone on the TG index was warranted.
In 9 studies, G. pentaphyllum alone was used as the experimental group, and 12 studies used combined drugs as the experimental group. After heterogeneity test, there was great heterogeneity between G. pentaphyllum group (Chi2 = 933.96, p < 0.01, I2 = 99%) and combination group (Chi2 = 430.15, p < 0.01, I2 = 97%). The random effect model was used for analysis. The results showed that the LDL reduction ability of G. pentaphyllum alone was stronger than that of the control group, but there was no statistical difference between the two groups (SMDalone = –1.33, 95% CI [–3.71, 1.05], p = 0.27 > 0.05). However, the ability of reducing LDL in the combination group was stronger than that in the control group, and the result was statistically different (SMDcombination = –1.38, 95% CI [–2.30, –0.0.45], p < 0.05). Due to the continued high heterogeneity observed, even after conducting subgroup analysis, a sensitivity analysis was performed by excluding the literature with the highest item heterogeneity within each subgroup. Subsequently, the exclusion of the two studies led to a shift in the trends observed in the subgroups and overall results (SMDcombination = –0.84, 95% CI [–1.69, 0.01], p = 0.05). These findings indicated that the conclusion reached was unstable and suggested the inclusion of relatively low-quality literature in the study. Therefore, it was essential to extensively discuss the impact of G. pentaphyllum on the LDL index.
Thirteen studies utilized G. pentaphyllum as the sole experimental group, whereas ten studies employed combined drugs as the experimental group. Following the heterogeneity test, significant heterogeneity was observed in both the G. pentaphyllum group (Chi2 = 200.32, p < 0.01, I2 = 94%) and the combination group (Chi2 = 174.21, p < 0.01, I2 = 95%). The random effect model was used for analysis. The results showed that the ability to increase HDL of G. pentaphyllum alone was stronger than that of the control group, and the difference was statistically significant (SMDalone = 1.17, 95% CI [0.57, 1.76], p < 0.05). The ability to increase HDL in the combination group was stronger than that in the control group, and the result was statistically different (SMDcombination = 1.72, 95% CI [1.04, 2.41], p < 0.01). Due to the persistent high heterogeneity even after conducting subgroup analysis, a sensitivity analysis was performed by excluding the literature with the highest item heterogeneity within each subgroup. After excluding the two studies, both the subgroup trends and overall results remained unchanged, indicating the stability of the data analysis.
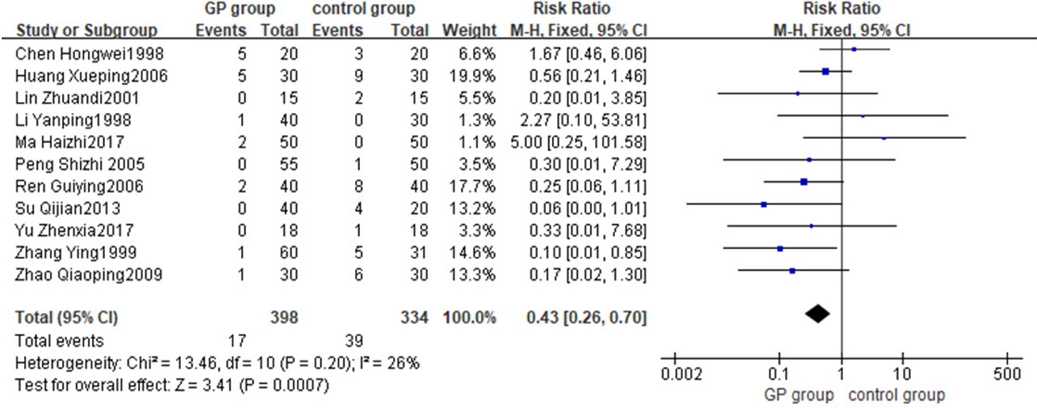
Adverse reactions were reported in 11 included studies, 7 reports showed adverse reactions in G. pentaphyllum experimental group and 9 studies showed adverse reactions in control group, details were shown in Table 7. According to the heterogeneity test, the heterogeneity included in the study was small (Chi2 = 13.46, p = 0.20, I2 = 26%) FEM was used for analysis. The results indicated that the incidence of adverse reactions in the G. pentaphyllum experimental group was lower than that in the control group. No elevation of transaminase levels was observed, demonstrating the minimal hepatotoxicity of G. pentaphyllum. The safety of G. pentaphyllum in the treatment of hyperlipidemia was found to be superior to conventional treatment, and this difference was statistically significant (RR = 0.43, 95% CI [0.26, 0.70], p < 0.01) (Fig. 2). These findings suggested the potential of G. pentaphyllum as an alternative medication for hyperlipidemia.
Among the four blood lipid evaluation indexes, only the HDL index demonstrates a superior effect of G. pentaphyllum alone compared to other drugs commonly used in clinical practice, and this difference was statistically significant (SMDHDL = 1.17, 95% CI [0.57, 1.76], p < 0.05). Consequently, an assessment of bias risk was conducted for the literature reporting HDL indices. Subsequently, the G. pentaphyllum treatment group was subjected to further subgroup analysis based on the type of hyperlipidemia. The results show that one study exhibits a small effect size and shows relatively apparent publication bias, while the remaining studies were evenly distributed on both sides of the funnel plot, reflecting a mildly inverted funnel shape (Fig. 3). Therefore, most of the literatures exhibited minimal risk of publication bias in relation to HDL indicators.
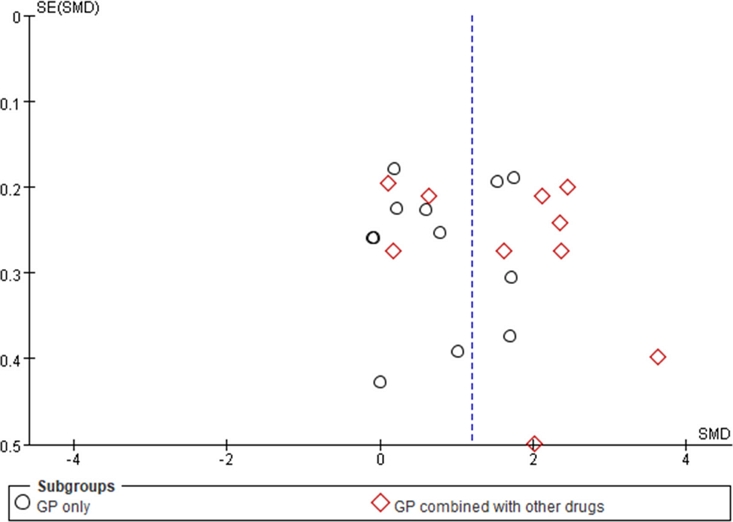
Funnel plot of risk assessment of publication offset of HDL index.Note: SE: Standard Error; SMD: standardized mean difference; other drugs: Inositol nicotinate, Nifedipine, energy mixture and diammonium glycyrrhizinate, Simvastatin, Metformin hydrochloride, Atto vastatin.
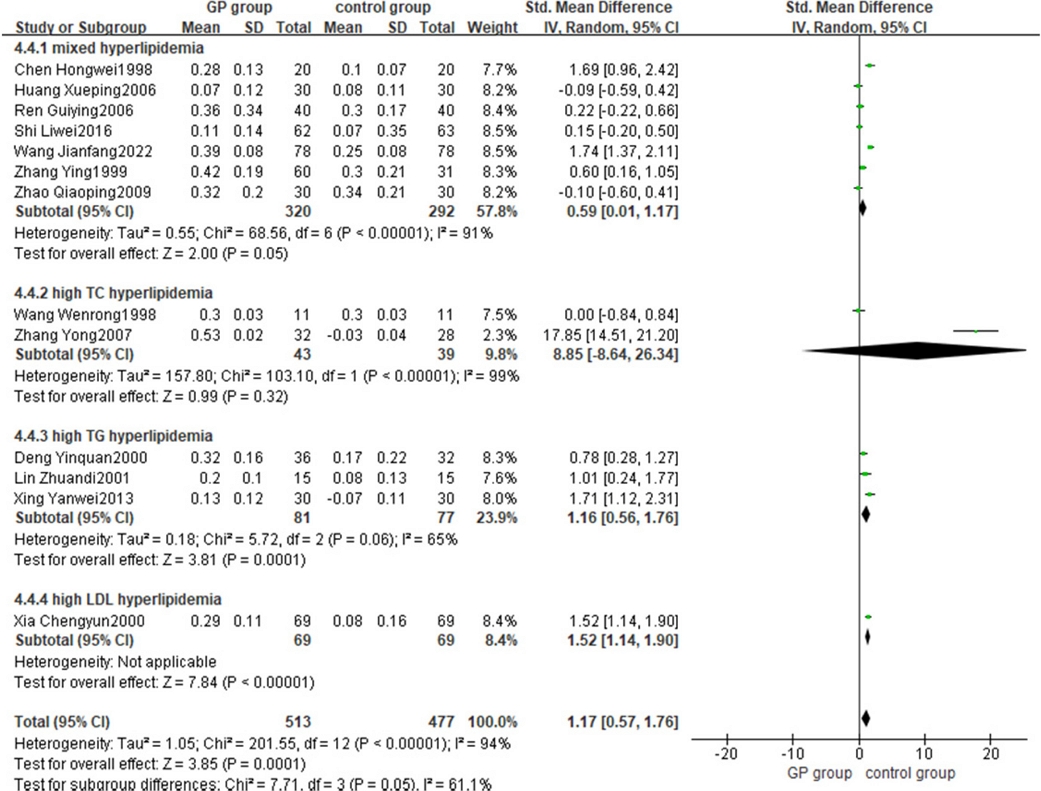
The results of the subsequent subgroup analysis revealed seven cases of mixed hyperlipidemia, two cases of high TC hyperlipidemia, three cases of high TG hyperlipidemia, and one case of high LDL hyperlipidemia. The results demonstrated that G. pentaphyllum monotherapy significantly increased the HDL index in patients with mixed dyslipidemia, hypercholesterolemia, and hypertriglyceridemia compared to conventional treatment. The effect size interval did not cross the zero point, indicating a significant difference between the G. pentaphyllum experimental group and the conventional treatment control group. (Fig 4).
This study conducted a meta-analysis of four clinical indicators, including TC, TG, LDL, and HDL. The study found that G. pentaphyllum alone can promote the generation of high-density lipoprotein, and its effect was superior to commonly used clinical drugs. Furthermore, it exhibited particularly good efficacy for hyperlipidemia with high triglyceride levels. The LDL, TC, and TG lowering effects of G. pentaphyllum used alone were on par with those of conventional lipid-lowering medications. Regarding hepatoprotection, G. pentaphyllum has the ability to decrease AST and ALT levels in hyperlipidemia patients. The study also discovered that G. pentaphyllum can enhance the lipid-lowering capabilities of conventional drugs. In conclusion, G. pentaphyllum has the potential as a natural remedy for treating hyperlipidemia.
There exist numerous research studies on the mechanisms of G. pentaphyllum in treating hyperlipidemia. One study confirmed that the total saponins extracted from the underground parts of G. pentaphyllum can inhibit the increase of peroxisome proliferator-activated receptor-γ (PPAR-γ) levels in the livers of rats fed a high-fat diet.11 The elevation of PPAR-γ was associated with the formation of adipocytes. G. pentaphyllum saponins can inhibit obesity in rats fed a high-fat diet. In our study, G. pentaphyllum total saponins were extracted from heated G. pentaphyllum and quantitatively analyzed.43 The preventive effect of G. pentaphyllum total saponins on high-fat diet-induced hyperlipidemia in mice was investigated. Further research demonstrated that heated G. pentaphyllum saponins (HGyp) can activate the SREBP/ACC/PPAR/LXRo signaling pathway, improving lipid metabolism disorders. Moreover, HGyp can regulate lipid metabolism disorders by altering the abundance of various lipid compounds such as DGs, TGS, CERs, PES, PSS, and PC.44 The mechanism of G. pentaphyllum in treating hyperlipidemia was complex and requires further comprehensive and in-depth research.
In the safety evaluation of G. pentaphyllum, a total of 11 studies encompassing 398 subjects were included. Among them, only 17 subjects reported adverse reactions upon taking G. pentaphyllum, and most of these reactions were mild gastrointestinal symptoms. These findings provide evidence of the low incidence of side effects and the high safety profile associated with G. pentaphyllum. Considering that hyperlipidemia primarily affects older middle-aged and elderly individuals who require long-term medication, the use of G. pentaphyllum as a long-term treatment option for controlling hyperlipidemia is a favorable choice, given its high safety profile.
Although our research results show that G. pentaphyllum has a positive effect on hyperlipidemia, there are still some limitations as follows: (1) In some of the included studies, random grouping was mentioned without providing a detailed explanation of the randomization method or reporting the concealment of random schemes. Furthermore, most of the literatures mentioned that patients signed informed consent forms and did not employ blind methods. These methodological shortcomings raise concerns about potential bias in the experimental results. (2) The number of included studies in this paper was limited, and the overall quality of the literature was deemed low. Additionally, a high level of heterogeneity was observed among the studies. These factors contribute to limitations in the robustness and generalizability of the findings. (3) The treatment duration documented in the literatures was relatively short, with the shortest duration being only 4 weeks. Considering that hyperlipidemia is a chronic disease, a longer treatment duration may be necessary to obtain a more comprehensive and realistic conclusion.
Conclusion
In this study, the clinical efficacy of G. pentaphyllum and gypenosides in the treatment of hyperlipidemia was analyzed. The results revealed that the G. pentaphyllum and gypenosides extract exhibited superior effects in increasing HDL levels. Furthermore, when combined with conventional lipid-lowering drugs, there was a noticeable enhancement. Additionally, it demonstrated a certain degree of liver protection, displayed good safety, and was found to be suitable for long-term use as a control measure for hyperlipidemia. However, it should be noted that the clinical research data included in this study appeared to be heterogeneous, and the overall quality of the literature was deemed low. Despite these limitations, this study provides valuable insights into the effectiveness of G. pentaphyllum in the clinical treatment of hyperlipidemia. Further research is warranted to refine our understanding of its therapeutic potential.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82274209).
Conflict of interest
The authors declare that they have no conflicts of interest.
References
-
Mandraffino, G.; Morace, C.; Franzè, M. S.; Nassisi, V.; Sinicropi, D.; Cinquegrani, M.; Saitta, C.; Scoglio, R.; Marino, S.; Belvedere, A.; Cairo, V.; Lo Gullo, A. L.; Scuruchi, M.; Raimondo, G.; Squadrito, G. Biomedicines 2022, 10, 1770.
[https://doi.org/10.3390/biomedicines10081770]

-
Gaggini, M.; Gorini, F.; Vassalle, C. Int. J. Mol. Sci. 2022, 24, 75.
[https://doi.org/10.3390/ijms24010075]

-
Hasson, T. S.; Said, E.; Helal, M. G. Life Sci. 2022, 306, 120790.
[https://doi.org/10.1016/j.lfs.2022.120790]

-
Park, C. M.; Min, O. J.; Kim, M. S.; Sharma, B. R.; Kim, D. W.; Rhyu, D. Y. Nat. Prod. Sci. 2022, 28, 161–167.
[https://doi.org/10.20307/nps.2022.28.4.161]

-
Chong, B.; Kong, G.; Shankar, K.; Chew, H. S. J.; Lin, C.; Goh, R.; Chin, Y. H.; Tan, D. J. H.; Chan, K. E.; Lim, W. H.; Syn, N.; Chan, S. P.; Wang, J. W.; Khoo, C. M.; Dimitriadis, G. K.; Wijarnpreecha, K.; Sanyal, A.; Noureddin, M.; Siddiqui, M. S.; Foo, R.; Mehta, A.; Figtree, G. A.; Hausenloy, D. J.; Chan, M. Y.; Ng, C. H.; Muthiah, M.; Mamas, M. A.; Chew, N. W. S. Metabolism 2023, 141, 155402.
[https://doi.org/10.1016/j.metabol.2023.155402]

-
Aguilar-Salinas, C. A.; Gómez-Díaz, R. A.; Corral, P. J. Clin. Endocrinol. Metab. 2022, 107, 1216–1224.
[https://doi.org/10.1210/clinem/dgab876]

-
Banach, M.; Mikhailidis, D. P. Cardiol. Clin. 2018, 36, 225–231.
[https://doi.org/10.1016/j.ccl.2017.12.004]

-
Hollingworth, S. A.; Ostino, R.; David, M. C.; Martin, J. H.; Tett, S. E. Cardiovasc. Ther. 2017, 35, 40–46.
[https://doi.org/10.1111/1755-5922.12236]

-
Coppinger, C.; Movahed, M. R.; Azemawah, V.; Peyton, L.; Gregory, J.; Hashemzadeh, M. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221100107.
[https://doi.org/10.1177/10742484221100107]

-
Weng, X.; Lou, Y. Y.; Wang, Y. S.; Huang, Y. P.; Zhang, J.; Yin, Z. Q.; Pan, K. Bioorg. Chem. 2021, 111, 104843.
[https://doi.org/10.1016/j.bioorg.2021.104843]

- Teng, F.; Li, X. W.; Li, M.; Fan, D. D.; Zhu, J. J.; Gao, H. M.; Wang, Z. M. China J. Chin. Mater. Med. 2022, 47, 5022–5031.
-
Li, S. G. Henan Trad. Chin. Med. 2015, 35, 1688–1689.
[https://doi.org/10.1108/IJOPM-01-2014-0014]

-
Tanner, M. A.; Bu, X.; Steimle, J. A.; Myers, P. R. Nitric Oxide 1999, 3, 359–365.
[https://doi.org/10.1006/niox.1999.0245]

-
Su, S.; Wang, J.; Wang, J.; Yu, R.; Sun, L.; Zhang, Y.; Song, L.; Pu, W.; Tang, Y.; Yu, Y.; Zhou, K. Phytother. Res. 2022, 36, 2982–2998.
[https://doi.org/10.1002/ptr.7493]

-
Shaito, A.; Thuan, D. T. B.; Phu, H. T.; Nguyen, T. H. D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G. K.; Eid, A. H.; Pintus, G. Front. Pharmacol. 2020, 11, 422.
[https://doi.org/10.3389/fphar.2020.00422]

- Ren, G. Y. Sichuan Med. J. 2006, 6, 606–607.
- Shi, L. W. Clinical Study on the Effect of Yun Pi Topng Xin Decoction for Hyperlipidemia; China Academy of Chinese Medical Sciences: China, 2016.
-
Wu, S. R.; Zuo, Z. C.; Yuan, L. J. Anhui Med. J. 1995, 16, 44.
[https://doi.org/10.1108/00438029510082567]

- Wu, J. H.; Lv, H. C.; Liu, C. Y. J. Heze Med. Coll. 2000, 12, 48–50.
- Xia, C. Y.; Liu, X. H.; Deng, L. Y.; Zhou, J. G. China J. Mod. Med. 2000, 10, 15–18.
- Sun, W. S.; Wu, X. L.; Qiao, C. L. J. Zhejiang Chin. Med. Univ. 2004, 28, 24–26.
- Zhang, Q. X.; Wang, W. Heilongjiang Med. J. 2011, 35, 37–39.
- Zhang, Y.; Zhang, J. E.; Wu, P. Y.; Zhang, Q. H. J. Yunyang Medical College 2007, 26, 199–202.
- Zhang, Y.; Hua, Q.; Sun, G. W. Chin. J. Inform. Tradit. Chin. Med. 1999, 6, 35–36.
- Peng, S. Z.; Deng, Y. Y.; Tang, Z. H. Clin. Focus. 2005, 20, 527–528.
-
Xu, J. H. J. Front. Med. 2013, 162–163.
[https://doi.org/10.1007/s10354-013-0204-6]

-
Zhu, H. M. Diet Health 2019, 6, 64–65.
[https://doi.org/10.18231/j.jchm.2019.014]

- Li, Y. Chin. J. Integr. Tradit. West. Med. Intensive Crit. Care 1998, 5, 56–57.
-
Lin, Z. Z. J. Guangdong Med. Coll. 2001, 19, 200–201.
[https://doi.org/10.1016/S0167-7799(01)01637-7]

- Wang, J. F.; Chen, Y. Y. New Chin. Med. 2022, 54, 117–120.
- Wang, W. R.; Huang, F. X.; Wu, Q. Fujian J. TCM 1998, 29, 12.
-
Shi, M.; Fang, W. Chin. J. Biochem. Pharma. 2016, 36, 134–137.
[https://doi.org/10.6023/cjoc201601023]

- Yu, Z. X. China Pract. Med. 2017, 12, 93–95.
- Su, Q. J.; Liang, F. L.; Li, Y. Z.; Deng, X.; Deng, M. H.; Zhang, Y. P.; Liu, Z. W. Chin. Gen. Pract. 2012, 15, 3903–3905.
- Su, Q. J.; Liang, F. L.; Li, Y. Z.; Deng, X.; Deng, M. H.; Zhang, Y. P.; Liu, Z. W. China Pharm. 2013, 24, 332–334.
- Zhao, Q. P.; Lu, Q. Y.; Lu, H. Y. J. Zhejiang Chin. Med. Univ. 2009, 33, 783–785.
- Deng, Y. Q.; Fan, X. F. Zhejiang J. Integr. Tradit. Chin. West. Med. 2000, 10, 522–524.
- Xing, Y. W.; Teng, F.; Gao, Y. H.; Xing, Y. H.; He, Q. Y. Chin. J. Integr. Med. Cardio/Cerebrovasc. Dis. 2013, 11, 655–657.
- Chen, H. W.; Wang, S. L.; Chen, Y. J. Med. Forum 1998, 6, 51–52.
- Ma, H. Z. Early interventions and traditional Chinese medical Syndrome analysis of Xuezhikang and gynostemma to nonalcoholic fatty liver disease with impaired glucose regulation; Guangzhou University of Chinese Medicine: China, 2017, pp 15–34.
- Huang, P.; Cheng, J. -L.; Zhang, L.; Lin, B. China J. Mod. Med. 2007, 17, 206–208.
-
Huang, X. P. China Pharm. 2006, 15, 46.
[https://doi.org/10.1016/j.visres.2006.01.031]

- Duan, Y.; Yang, J.; Xie, J. B.; Xie, P.; Qi, Y. S.; Zhao, M. T.; Piao, X. L. China J. Chin. Mater. Med. 2021, 46, 5314–5319.
-
Xie, P.; Xie, J. B.; Xiao, M. Y.; Guo, M.; Qi, Y. S.; Li, F. F.; Piao, X. L. Phytomedicine 2023, 115, 154834.
[https://doi.org/10.1016/j.phymed.2023.154834]