Chemical Constituents from the Leaves of Dischidia nummularia (Asclepiadaceae)
Abstract
The investigation of chemical constituents from Dischidia nummularia leaves, which belongs to the Asclepiadaceae family, resulted in five known compounds. Three compounds were suggested as triterpenoid derivatives, namely glutinol (1), α-amyrin (2) and friedelin (3). In addition, two other compounds were a steroid derivative (4), i.e., stigmast-5-ene-3β-ol (4) and a xanthone derivative, i.e., α-mangostin (5). The chemical structures of 1–5 were elucidated based on detailed analysis of NMR (1D and 2D) spectroscopic data and compared with literature data. Interestingly, compounds 1 and 5 were first obtained from the Asclepiadaceae family, while compound 2 was isolated for the first time from the Dischidia genus. The acetone crude extract and compounds 1–3 showed weak cytotoxicity against murine leukemia P-388 cells. After all, the current study is the first report of a phytochemical study of D. nummularia.
Keywords:
Dischidia nummularia, Triterpenoid, Steroid, Xanthone, CytotoxicityIntroduction
Dischidia plant, a type of epiphytic living abundantly in other plants as the host, is one of the genera of the milkweed family group (Asclepiadaceae), which consists of 120 species.1,2 Generally, Dischidia are herbs and small shrubs as well. They are native to the tropics and subtropics regions2,3 and are widely distributed in China, Taiwan, India, Bangladesh, and Southeast Asia with three non-endemic species occurring in the East Coast tropics of Australia.4 For decades, organic extracts from Dischidia have been reported to have biological activities such as anticancer,5 antiproliferative,6 anti-inflammatory3,7,8 and antioxidant.5,10 Furthermore, previous phytochemistry research on this genus revealed the presence of various compounds, including triterpenes (friedelin (4), friedelinol, dischidiol, glutinone, β-amyrin acetate, taraxerol, 3β,6β, 19α,23-tetrahydroxyurs-12-en-28-oic acid, 3β,6α,19α,23-tetrahydroxyurs-12-en-28-oic acid and 6β,19α,22α-trihydroxyurs-12-en-3-oxo-28-oic acid), steroids (disformone and sitosteryl-3-O-β glucopyranoside), and flavonoids (kaempferol, isorhamnetin and quercetin).3,9 One species of Dischidia is D. nummularia, which is known as a herb epiphytic (length of 10–50 cm) with a divided part and a smooth stem. It is distributed in Srilanka, India, Bangladesh, Australia, and Southeast Asia,11 including Indonesia (throughout). In Kupang, Indonesia, the boiled water of the leaves of the plant are used as folk medicine to treat cysts, cervical cancer, heart disease, gonorrhea, apthae tropicae (tropical sores) and to relieve the pain of stings from Ikan Sembilang (a spiny catfish type). Thus far, based on our best knowledge, the chemical constituents of D. nummularia have not yet been reported. It is the important point of this research to evaluate the chemical constituents of this plant and its bioactivity. Therefore, the isolation of the compounds as well as cytotoxic evaluation of the crude extract and isolated compounds (1–5) from D. nummularia leaves are described herein.
Experimental
General experimental procedures – 1H and 13C NMR spectra were recorded on a spectrometer of Agilent DD2 system, operating at 500 (1H) and 125 (13C) MHz, using CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard. Optical rotations were measured using an Autopol IV polarimeter (Rudolph Research Analytical, New Jersey). Melting points were measured on an Electrothermal melting point 110 V. Vacuum liquid chromatography (VLC) and centrifugal planar chromatography (CPC) were carried out using Merk Si gel 60 GF254 art.7731 and Si gel PF254 art.7749, respectively. Gravitational column chromatography (GCC) was carried out using Merk Si gel G60 35–70 mesh (art.7734) and Sephadex LH-20 (Amersham Pharmacia Biotech). Thin layer chromatography (TLC) analyses used precoated Si gel plates (Merk Kieselgel 60 GF254, 0.25 mm thickness, art.5554).
Plant materials – The leaves of D. nummularia were collected from Kupang, Timor Island, East Nusa Tenggara Province, Indonesia, in March 2016. The plant was determined and deposit at Herbarium Bogoriense, Research Center for Biology, Indonesian Institute of Sciences with voucher specimen BO-1274278.
Extraction and isolation – The dried leaves powdered of D. nummularia (1 kg) were extracted at room temperature with acetone three times (each 24 h) to give a dark yellow crude extract (200 g). Part of the crude extract (40 g) was fractionated using VLC and eluted with the gradient polarity solvent system, i.e., n-hexane 100%, n-hexane-CH2Cl2 (9:1 and 1:1), CH2Cl2 100%, CH2Cl2-EtOAc (1:1 and 1:4), EtOAc 100%, acetone 100% and MeOH 100% to afford 9 fractions (F1–F9). Fraction F3 (1.1 g) was refractionated using the same method and eluted with n-hexane-CH2Cl2 (7:3, 6:4, 4:6, and 2:8), CH2Cl2 100%, acetone 100% and MeOH 100% to yield 5 subfractions (F3.1–F3.5). Subfraction F3.1 (389 mg) was subjected to CPC eluted with n-hexane-EtOAc (12:1) to give 4 fractions (F3.1.1–F3.1.4). Fraction F3.1.3 (147 mg) was purified by GCC using Sephadex LH-20 (MeOH) to afford compound 1 (15 mg). Next, fraction F3.2 (100 mg) was separated by CPC eluted with n-hexane-EtOAc (15:1) to obtain compound 2 (5 mg). Meanwhile, fraction F4 (2.0 g) was purified by Si gel GCC (n-hexane-CHCl3, 9:1) to afford compound 3 (15 mg). Fraction F7 (10 g) was also separated by VLC eluted with n-hexane-CH2Cl2 (1:1, 3:7, and 1:9), CH2Cl2 100%, CH2Cl2-acetone (9:1), acetone 100% and MeOH 100% into 8 fractions (F7.1–F7.8). Subfraction F7.4 (148 mg) was also subjected to a GCC using Sephadex LH-20 (MeOH) to give compound 4 (22 mg). Furthermore, subfraction F7.6 (1.2 g) was further purified by CPC (n-hexane-EtOAc, 8:2) to obtain compound 5 (15 mg).
Glutinol (1) - Colorless crystals; mp 208–210oC; : +58.5 (c 9 × 10–4, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 3.46 (1H, t, H-3), 5.62 (1H, m, H-6), 1.04 (3H, s, H-23). 1,14 (3H, s, H-24), 0.85 (3H, s, H-25), 1.01 (3H, s, H-26), 1.10 (3H, s, H-27), 1.16 (3H, s, H-28), 0.99 (3H, s, H-29), 0.95 (3H, s, H-30); 13C NMR (CDCl3, 125 MHz): δ 18.2 (C-1), 27.8 (C-2), 76.3 (C-3), 40.8 (C-4), 141.4 (C-5), 122.0 (C-6), 23.7 (C-7), 47.4 (C-8), 34.8 (C-9), 49.7 (C-10), 34.5 (C-11), 30.2 (C-12), 39.3 (C-13), 37.8 (C-14), 32.1 (C-15), 36.0 (C-16), 30.1 (C-17), 43.1 (C-18), 35.1 (C-19), 28.2 (C-20), 33.1 (C-21), 39.0 (C-22), 25.5 (C-23), 29.0 (C-24), 16.2 (C-25), 19.6 (C-26), 18.4 (C-27), 32.03 (C-28), 32.4 (C-29), 34.5 (C-30).
α-Amyrin (2) - White solid; mp 183–185oC; : +54.1 (c 8 × 10–4, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 3.15 (1H, H-3), 0.67 (1H, H-5), 5.12 (1H, H-12), 0.94 (3H, s, H-23), 0.76 (3H, s, H-24), 0.73 (3H, s, H-25), 0.89 (3H, s, H-26), 1.01 (3H, s, H-27), 0.95 (3H, s, H-28), 0.85 (3H, d, J = 6.0 Hz, H-29), 0.75 (3H, d, J = 7.0 Hz, H-30); 13C NMR (CDCl3, 125 MHz): δ 38.7 (C-1), 28.7 (C-2), 79.0 (C-3), 38.5 (C-4), 55.1 (C-5), 18.3 (C-6), 32.8 (C-7), 40.0 (C-8), 47.6 (C-9), 36.8 (C-10), 23.3 (C-11), 124.3 (C-12), 139.5 (C-13), 42.0 (C-14), 27.2 (C-15), 26.5 (C-16), 33.7 (C-17), 58.9 (C-18), 39.6 (C-19), 39.5 (C-20), 31.2 (C-21), 41.4 (C-22), 28.0 (C-23), 15.5 (C-24), 15.6 (C-25), 16.8 (C-26), 23.2 (C-27), 28.0 (C-28), 17.4 (C-29), 21.3 (C-30).
Friedeline (3) - Colorless crystal; mp 259–263oC; : –21.0 (c 5.1 × 10–1, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 1.67 (1H, m, H-1a), 1.96 (1H, m, H-1b), 2.23 (1H, m, H-2a), 2.39 (1H, m, H-2b), 2.23 (1H, m, H-4), 1.27 (1H, m, H-6a), 1.74 (1H, m, H-6b), 1.33 (1H, m, H-7a), 1.45 (1H, m, H-7b), 1.37 (1H, m, H-8), 1.50 (1H, m, H-10), 1.53 (1H, m, H-18), 0.94 (1H, m, H-22a), 1.48 (1H, m, H-22b), 0.86 (3H, d, J = 6.0 Hz, H-23), 0.70 (3H, s, H-24), 0.85 (3H, s, H-25), 0.98 (3H, s, H-26), 1.03 (3H, s, H-27), 1.16 (3H, s, H-28), 0.93 (3H, s, H-29), 0.98 (3H, s, H-30); 13C NMR (CDCl3, 125 MHz): δ 22.3 (C-1), 41.5 (C-2), 213.3 (C-3), 58.2 (C-4), 42.2 (C-5), 41.3 (C-6), 18.2 (C-7), 53.1 (C-8), 37.4 (C-9), 59.4 (C-10), 35.6 (C-11), 30.5 (C-12), 38.3 (C-13), 39.7 (C-14), 32.4 (C-15), 36.0 (C-16), 30.0 (C-17), 42.8 (C-18), 35.3 (C-19), 28.2 (C-20), 32.7 (C-21), 39.2 (C-22), 6.8 (C-23), 14.7 (C-24), 17.9 (C-25), 20.3 (C-26), 18.7 (C-27), 32.1 (C-28), 31.8 (C-29), 35.0 (C-30).
Stigmast-5-ene-3β-ol (4) - White solid; mp 135–138oC; : –20.0 (c 25 × 10–4, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 3.51 (1H, m, H-3), 2.21 (2H, H-4), 2.26 (2H, H-4), 5.33 (1H, dd, J = 1.8; 4.8 Hz, H-6), 0.66 (3H, s, H-18), 0.99 (3H, s, H-19), 0.90 (3H, d, J = 6.6 Hz, H-21), 0.79 (3H, d, J = 6.6 Hz, H-26), 0.82 (3H, d, J = 6.6 Hz, H-27), 0.86 (3H, t, J = 7.2 Hz, H-29); 13C NMR (CDCl3, 125 MHz): δ 37.38 (C-1), 32.03 (C-2), 71.96 (C-3), 42.45 (C-4), 140.87 (C-5), 121.87 (C-6), 31.78 (C-7), 32.05 (C-8), 50.25 (C-9), 36.29 (C-10), 21.22 (C-11), 39.90 (C-12), 42.41 (C-13), 56.89 (C-14), 24.45 (C-15), 28.40 (C-16), 56.17 (C-17), 12.12 (C-18), 19.16 (C-19), 36.64 (C-20), 18.92 (C-21), 34.07 (C-22), 26.16 (C-23), 45.95 (C-24), 29.26 (C-25), 19.97 (C-26), 19.55 (C-27), 23.19 (C-28), 12.00 (C-29).
α-Mangostin (5) - Yellow oil; 1H NMR (CDCl3, 500 MHz): δ 13.76 (1H, s, 1-OH), 9.50 (1H, s, 3-OH), 6.30 (1H, s, H-4), 6.82 (1H, s, H-5), 9.45 (1H, s, H-6), 3.43 (2H, d, J = 6.0 Hz, H-11), 5.26 (1H, m, J = 6.0 Hz, H-12), 1.83 (3H, s, H-14), 1.69 (3H, s, H-15), 4.08 (2H, d, J = 6.0 Hz, H-16), 5.26 (1H, m, J = 6.0 Hz, H-17), 1.76 (3H, s, H-19), 1.83 (3H, s, H-20), 3.80 (3H, s, 7-OCH3); 13C NMR (CDCl3, 125 MHz): δ 160.7 (C-1), 108.7 (C-2), 161.7 (C-3), 93.3 (C-4), 155.1 (C-4a), 101.7 (C-5), 155.8 (C-6), 142.6 (C-7), 137.1 (C-8), 112.3 (C-8a), 182.1 (C-9), 103.7 (C-9a), 154.7 (C-10a), 21.5 (C-11), 121.6 (C-12), 132.2 (C-13), 18.0 (C-14), 25.6 (C-15), 26.9 (C-16), 123.2 (C-17), 135.6 (C-18), 25.9 (C-19), 18.3 (C-20), 62.1 (O-CH3).
Cytotoxicity assay - The crude extract and compounds 1–3 were evaluated for their cytotoxicity against murine leukemia P-388 cells using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] assay as previously reported.12–14 The cells were seeded in 96-well plates (cell density of 3 × 104 cells/cm3). The crude extract and each compound (1–3) were added in various concentrations and incubated for 48 hours, where the crude extract and compounds were dissolved in dimethyl sulfoxide (DMSO). After 48 hours of incubation, 10 µL MTT reagent was added to each sample and then incubated for 4 hours. The MTT-stop solution containing sodium dodecyl sulfate (SDS) was added and the incubation continued for 24 hours. Optical density was read with a microplate reader at 550 nm. IC50 values were taken from the plotted graph of the percentage of live cells compared with control. MTT solution and DMSO (without cells and media) were taken as negative controls, while artonin E was used as a positive control.14,15
Results and Discussion
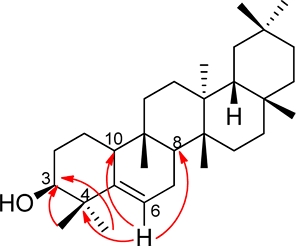
The leaves of D. nummularia were collected from Timor Island, East Nusa Tenggara, Indonesia. Then, the dry leaves powdered were extracted with acetone. Separation and purification of acetone extract using several chromatography techniques, including vacuum liquid chromatography (VLC), gravitational column chromatography (GCC) and centrifugal planar chromatography (CPC), yielded compounds 1–5 (Fig. 1).
All chemical structures of isolated compounds were determined by an extensive analysis of NMR (1D and 2D) spectroscopic data and by comparison with previously reported data. Compounds 1–5 were identified as glutinol (1),16 α-amyrin (2),17 friedelin (3),18 stigmast-5-ene-3β-ol or β-sitosterol (4)19 and α-mangostin (5).20 The isolation of all compounds (1–5) from D. nummularia was investigated for the first time in this study. Moreover, to our knowledge, compounds 1 and 5 were first isolated from the Asclepiadaceae family. In addition, the finding of compound 2 was first reported in the Dischidia genus.
Compound 1 was isolated as colorless crystals with : +58.5 (c 9 × 10–4, CHCl3), and melting point of 208–210oC, which was identified as glutinol, a triterpenoid. The 1H NMR spectrum of 1 exhibited a total of eight methyl protons signals at δH 0.85 (3H, s, H-25), 0.95 (3H, s, H-30), 0.99 (3H, s, H-29), 1.01 (3H, s, H-26), 1.04 (3H, s, H-23), 1.10 (3H, s, H-27), 1.14 (3H, s, H-24) and 1.16 (3H, s, H-28). Moreover, also showed the deshielded proton signal at δH 3.46 (1H, t, H-3) was assigned to oxygenated methine proton, which was directly attached to a hydroxyl group and one olefin methine proton at δH 5.26 (1H, m, H-6). The 13C NMR spectrum indicated a total of 30 carbon signals containing an oxygenated methine carbon which is hydroxyl group at δC 76.3 (C-3), an olefin quaternary carbon at δC 141.4 (C-5) and olefin methine carbon at δC 122.0 (C-6), eight methyl carbons at δC 25.5 (C-23), 29.0 (C-24), 16.2 (C-25), 19.6 (C-26), 18.4 (C-27), 32.03 (C-28), 32.4 (C-29), 34.5 (C-30); ten methylene carbons at δC 18.2 (C-1), 27.8 (C-2), 23.7 (C-7), 34.5 (C-11), 30.2 (C-12), 32.1 (C-15), 36.0 (C-16), 35.1 (C-19), 33.1 (C-21), 39.0 (C-22); three methine carbons at δC 47.4 (C-8), 49.7 (C-10) and 43.1 (C-18); and six quaternary carbons at δC 40.8 (C-4), 34.8 (C-9), 39.3 (C-13), 37.8 (C-14), 30.1 (C-17) and 28.2 (C-20).
Careful investigation by the 1H NMR and 13C NMR spectroscopic data established that compound 1 was glutinol, an triterpenoid type with C-3 oxidized to a hydroxyl and double bond at C-5 and C-6 that was confirmed by data comparison with those in the literatures16 and selected HMBC correlation (Fig. 2) of methyl proton at δH 1.04 (3H, s, H-23) and 1.14 (3H, s, H-24) with oxymethine carbon (C-3). Another correlation showed by olefin methine proton at δH 5.26 (1H, m, H-6) with quaternary carbon (C-4) and two units of methine carbon (C-8 and C-10).
Additionally, all isolated compounds have been reported as having various biological acivities. Compound 1 showed strong cytotoxic toward human ovarian cancer cell (OVACAR3 cell) with IC50 value of 6 µM21 and anti-inflammatory activity with active IC50 value of 2.86 µM.22 Compound 2 was have significant anticancer activity on four cancer cell lines (MCF-7, BEL-7402, SPC-A-1 and SGC-7901) with IC50 values of 7.2 ± 0.12, 8.2 ± 0.29, 7.6 ± 0.06 and 5.0 ± 0.12 µM23 and showed antimicrobial activity against S. aureus, E. Coli, S. pneumoniae, S. typhi and C. albicans with the same MICs and MBCs at 0.37 and 0.75 µg/mL, respectively.24 Compound 3 revealed significant inhibition (89.64%) of castor oil-induced diarrhea25 and also identified as the best possible inhibitor against prostate cancer CYP17A1 which was tested on hormone-sensitive (22Rv1) and insensitive (DU145) cell lines with the IC50 values were found to be 72.025 and 81.766 µg/mL, respectively.26 Compound 4 was shown to inhibit cells growth toward T47D, MCF-7, HeLa and WiDr cell lines with IC50 values of 0.55, 0.87, 0.76 and 0.99 µM, respectively27 and exhibited significantly antidiabetic activity at all glucose concentrations with minimum increase 66.48% at 10 µg/mL and maximum increase 74.46% at 80 µg/mL glucose concentration.28 Then compound 5 showed antibacterial activity against P. acnes and S. aureus with the same MICs and MBCs at 6.25 and 50 µg/mL, respectively;29 strong antimalarial activity with cytotoxic effect against the resistant plasmodium falciparum with IC50 value of 0.2 ± 0.01 µM;30 promising antiviral activity where exposure of compound 5 caused 55%, 48%, 50% and 47% reduction of infected cells when HepG2 cells were infected with DENV-1, DENV-2, DENV-3 and DENV-4, respectively;30 anti-inflammatory activity by diminished the LPS expressions in ocostatin M signaling through the mitogen-activated protein kinase (MAPK) pathways30 and potential anticancer activity by the cell growth inhibition of human leukemia cell line HL60 at IC50 value of 10 µM.31
Nonetheless, the present studi was the first time to investigate the cytotoxic activity of acetone crude extract and isolates (1–3) of D. nummularia leaves against murine leukemia P-388 cells according to the method of the MTT assay as described earlier.12,14 Weak cytotoxicity was exhibited by the acetone crude extract with IC50 value of 58.33 µg/mL and compounds 1–3 with IC50 values of 69.88, >234.74 and 216.17 µM, respectively (Table 1). Interestingly, compounds 4 and 5 were previously reported as active with IC50 values of 0.57 and 7.80 µM.32,33 Although the extract as well as compounds 1–3 were not active to the murine leukemia P-388 cells, but compounds 4 and 5 have been reported to be active against murine leukemia P-388 cells. In addition, compounds 1–3 that were not active in the murine leukemia P-388 cells have also reported to have potential activity against other various bioactivities as described before. Furthermore, more studies regarding the other bioactivities profile of the extract as well as chemical constituents of this plant are required.
Acknowledgments
We would like to thank the Indonesia Endowment Fund for Education (LPDP) scholarship of the Ministry of Finance, Indonesia for giving financial support to the first author. We are grateful to Prof. Dr. Yana Maolana Syah and Dr. Elvira Hermawati at Institut Teknologi Bandung for helping with NMR measurements.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Rahman, M. A.; Wilcock, C. C. J. Biogeography 1991, 18, 51–58.
[https://doi.org/10.2307/2845619]
- Hajari, Y.; Sulistijorini.; Ariyanti, N. S. J. Trop. Life Sci. 2018, 8, 269–278.
-
Chen, Z. S.; Lee, G. H.; Kuo, Y. H. Phytochemistry 1993, 34, 783–786.
[https://doi.org/10.1016/0031-9422(93)85359-Y]
-
Forster, P. I.; Liddle, D. J. Austrobaileya 1988, 2, 507–514.
[https://doi.org/10.5962/p.365729]
- Nurrani, L.; Ady, S.; Sugio, K. Antioxidant Activities of Four Medicinal Plants Utilization by Tribes in North Sulawesi; International Seminar Forest and Medicinal Plants of Manado: Indonesia, 2013.
- Khalil-ur-Rehman, M.; Wenzig, E. M. P.; Kretschmer, N.; Kunert, O.; Hofer, F.; Bauer, R. Planta Med. 2019, 85, 1525–1526.
- Wuyang, H. Traditional Chinese Medicinal Plants and Their Endophytic Fungi: Isolation, identification, and bioassay; The University of Hong Kong: China, 2008.
- Yang, L.; Wang, X. M.; Yu, B. L.; Yao, G. W.; Li, X. L.; Chen, J. B.; Xie, R. China Trop. Med. 2015, 15, 1302–1305.
-
Ma, X.; Yang, C.; Zhang, Y. Magn. Reson. Chem. 2008, 46, 571–575.
[https://doi.org/10.1002/mrc.2212]
- Tari, Y.; Cunha, T. D.; Lulan, T. Y.; Buang, Y. J. App. Chem. Sci. 2012, 1, 39–46.
-
Silalahi, M.; Nisyawati.; Walujo, E. B.; Supariatna, J.; Mangunwardoyo, W. J. Ethnopharmacol. 2015, 175, 432–443.
[https://doi.org/10.1016/j.jep.2015.09.009]
-
Sahidin.; Hakim, E. H.; Juliawaty, L. D.; Syah, Y. M.; Din, L. B.; Ghisalberti, E. L.; Latip, J.; Said, I. M.; Achmad, S. A. Z. Naturforsch. C. J. Biosci. 2005, 60, 723–727.
[https://doi.org/10.1515/znc-2005-9-1011]
-
Yang, R. L.; Yan, Z. H.; Lu, Y. J. Agric. Food Chem. 2008, 56, 3024–3027.
[https://doi.org/10.1021/jf7036517]
- Riga, R.; Happyana, N.; Hakim, E. H. J. Appl. Pharm. Sci. 2019, 9, 108–124.
-
Hakim, E. H.; Achmad, S. A.; Juliawaty, L. D.; Makmur, L.; Syah, Y. M.; Aimi, N.; Kitajima, M.; Takayama, H.; Ghisalberti, E. L. J. Nat. Med. 2007, 61, 229.
[https://doi.org/10.1007/s11418-006-0106-7]
- Rosandy, A. R.; Din, L. B.; Yaacob, W. A.; Yusoff, N. I.; Sahidin, I.; Latip, J.; Nataqain, S.; Noor, N. M. Malaysian J. Anal. Sci. 2013, 17, 50–58.
- Vazquez, L. H.; Palazon, J.; Navarro-Ocaña, A. Phytochemicals 2012, 23, 487–502.
-
Rodrigues, P. M.; Gomes, J. V. D.; Jamal, C. M.; Neto, A. C.; Santos, M. L.; Fagg, C. W.; Fonseca-Bazzo, Y. M.; Magalhaes, P. O.; de Sales, P. M.; Silveira, D. Food Chem. Toxicol. 2017, 109, 1063–1068.
[https://doi.org/10.1016/j.fct.2017.05.026]
- Katiyar, D.; Singh, V.; Gilani, S. J.; Goel, R.; Vats, A.; Bajaj, U. Int. J. Drug Dev. Res. 2014, 6, 92–98.
-
Ha, L. D.; Hansen, P. E.; Vang, O.; Duus, F.; Pham, H. D.; Nguyen, L. H. D. Chem. Pharm. Bull (Tokyo). 2009, 57, 830–834.
[https://doi.org/10.1248/cpb.57.830]
-
Chen, Y.; Li, J. Trop. J. Pharm. Res. 2021, 20, 1331–1335.
[https://doi.org/10.4314/tjpr.v20i7.2]
-
Adebayo, S. A.; Shai, L. J.; Eloff, J. N. Asian Pac. J. Trop. Med. 2017, 10, 42–46.
[https://doi.org/10.1016/j.apjtm.2016.12.004]
- Abu-Lafi, S.; Rayan, B.; Kadan, S.; Abu-Lafi, M.; Rayan, A. Oncol. Lett. 2019, 17, 713–717.
-
Ekalu, A.; Ayo, R. G. O.; Habila, J. D.; Hamisu, I. JOTCSA 2019, 6, 411–418.
[https://doi.org/10.18596/jotcsa.571770]
-
Antonisamy, P.; Duraipandiyan, V.; Ignacimuthu, S.; Kim, J. H. South Indian J. Bio. Sci. 2015, 1, 34–37.
[https://doi.org/10.22205/sijbs/2015/v1/i1/100440]
-
Joshi, B. P.; Bhandare, V. V.; Vankawala, M.; Patel, P.; Patel, R.; Vyas, B.; Krishnamurty, R. J. Biomol. Struct. Dyn. 2022, 14, 1–26.
[https://doi.org/10.1080/07391102.2022.2145497]
-
Hasibuan, P. A. Z.; Sitorus, P.; Satria, D. Asian J. Pharm. Clin. Res. 2017, 10, 306–308.
[https://doi.org/10.22159/ajpcr.2017.v10i5.16931]
- Zeb, M. A.; Khan, S. U.; Rahman, T. U.; Sajid, M.; Seloni, S. Pharm. Pharmacol. Int. J. 2017, 5, 204–207.
-
Tanngoen, P.; Lamlertthon, S.; Tiyaboonchai, W. Int. J. Pharm. Pharma. Sci. 2019, 11, 45–49.
[https://doi.org/10.22159/ijpps.2019v11i6.32927]
-
Chen, G.; Li, Y.; Wang, W.; Deng, L. Expert Opin. Ther. Pat. 2018, 28, 415–427.
[https://doi.org/10.1080/13543776.2018.1455829]
-
Muchtaridi, M.; Wijaya, C. A. Asian J. Pharm. Clin. Res. 2017, 10, 440–445.
[https://doi.org/10.22159/ajpcr.2017.v10i12.20812]
- Nurhamidah, N. Characterization of Secondary Metabolites and Cytotoxic and Antibacterial Activities from Fruit of Ficus aurata (Miq.); Universitas Andalas Padang: Indonesia, 2016.
- Hermawati, E. Xanthone from Fruit Skin of Garcinia mangostana (Guttiferae) and Its Bioactivity as Anti-tumor: Master Thesis; Institut Teknologi Bandung; Indonesia, 2011.