
Phytochemical Characterization, Antioxidant and Antimicrobial Activities of Calicotome villosa Link from Morocco
Abstract
In this research, we evaluated the antioxidant and antimicrobial activities of hydroalcoholic and aqueous extracts from four distinct parts of Calicotome villosa from Morocco (flowers, leaves, stems and roots). Quantification of total polyphenols by the Folin-Ciocalteu method and flavonoids by the aluminum trichloride method revealed variable concentrations. The hydroalcoholic leaf extracts showed the highest concentrations of total polyphenols (35.21 mg GAE/g extract) and flavonoids (58.67 mg QE/g extract), while the hydroalcoholic root extract showed the highest content of condensed tannins, determined by the vanillin method. The antioxidant activity of extracts was assessed using three complementary methods; DPPH free radical scavenging, iron reduction (FRAP) and total antioxidant capacity (TAC). The IC50 obtained for the DPPH test ranged from 0.05 mg/mL (for hydroalcoholic leaf extracts) to 0.41 mg/mL (for aqueous root extracts), showing a lower free radical scavenging activity than ascorbic acid (0.001 mg/mL). According to the FRAP method, the leaf fraction showed a higher reducing power than other parts of the plant, although slightly lower than that of ascorbic acid. Compounds in root and leaf extracts have a significant total antioxidant capacity, followed by stems and flowers, in the order of 0.34 ± 0.07 (hydroalcoholic roots) and 0.30 ± 0.06 (hydroalcoholic leaves) mg EAA/g extract. As regards antimicrobial activity, hydroalcoholic extracts showed significant inhibition against several multi-resistant bacterial strains, such as Staphylococcus aureus, Dickey asolani, Pectobacterium wasabiae and Pectobacterium brasiliensis, but no effect was observed against Candida albicans across all extracts tested.
Keywords:
Calicotome villosa, Antioxidant, Antimicrobial, Phytochemical characterizationIntroduction
Since ancient times, medicinal plants have been sought after for their potential as a source of natural remedies. Their applications in the treatment of various infectious diseases have long been studied.1 The therapeutic properties of these plants are mainly attributed to their secondary metabolites, natural bioactive compounds present in large quantities in various organs and sometimes in specialized cells.
Calicotome villosa (Poir.) Link (Fabaceae), also known as “El Gendoul” in Arabic, is a shrub that can reach 2 meters in height. It is characterized by gray-tomentose stems terminating in spikes, urban pods and trifoliate, oval leaves. Its clustered yellow flowers bloom in spring.2,3 This plant is widespread in the Mediterranean region, particularly in cool areas. C. villosa (Poir.) Link subsp. intermedia is a thorny shrub 50 to 150 cm high, with yellow flowers in spring, mainly found in North Africa and Spain.4 In Sicilian folk medicine, it is recognized for its anti-tumoral properties and its effectiveness in the treatment of boils, skin abscesses and chilblains.5,6
Plant products are rich reservoirs of various biologically active compounds, mainly phenolics. Studies have shown that these phytochemicals possess a diverse range of biological properties, including antioxidant and antimicrobial activities.1
Previous investigations on chemical composition of C. villosa have led to the identification of several compounds such as flavonols and alkaloids from the seeds7, flavones glucosides, alkaloids and anthraquinones from the leaves and flowers2, flavones glucosides, steroids and chalcone from the stems.8
Natural antioxidants such as polyphenols, flavonoids and their derivatives play a crucial role in inhibiting oxidative damage caused by reactive oxygen and nitrogen species present in biological systems. Their importance is paramount in the prevention of various human diseases such as cardiovascular disease, cancer and diabetes, as well as neurodegenerative diseases such as Parkinson’s and Alzheimer’s.9–10
In recent years, drug resistance in human pathogenic microorganisms has increased due to the excessive use of conventional antimicrobials. This phenomenon has prompted researchers to explore new antimicrobial substances from a variety of sources, including medicinal plants, which represent a promising source of novel antimicrobial agents.11
The present study focuses on the extraction and quantification of polyphenolic compounds, evaluating their antioxidant and antimicrobial activity against different microbial strains.
Experimental
General experimental procedures – Ammonium molybdate was purchased from Honeywell (India). Sodium phosphate was from Oxford (Maharashtra, India). n-Hexane, methanol, aluminum chlorure, gallic acid, sodium acetate, HCl, catechol, DPPH (2,2-diphenyl-1-picrylhydrazyl), potassium ferricyanide (1% K3Fe (CN)6), trichloroacetic acid, ferric chloride (FeCl3), vanillin, quercetin and sodium carbonate were from Carlo Erba Reagents (Val de Reuil Cedex, France). Sulphuric acid was from VWR chemicals (France). Folin-Ciocalteu reagent was from Oxford Lab (Maharashtra, India) and ethanol was from Sharlau (Barcelona, Spain).
Plant material – The plant studied in this research is C. villosa, collected in the Fez-Meknes region of Morocco during the flowering period in March 2021, at El Menzel (geographic coordinates: 34.61678o N, −4.17824o E). Botanical identification was carried out by Professor Amina Bari of the Biology Department of the Dhar El Mahraz Faculty of Sciences at the University Sidi Mohamed Ben Abdelah-Fez. The plant parts (leaves, flowers, stems and roots) were washed, dried in the dark for several days, then ground to a fine powder using an electric blender. This powder was stored in glass bottles until further use.
Preparation of the aqueous and hydroalcoholic extracts – The extraction of active plant ingredients by decoction is a widely used traditional method. It involves heating plant parts in a solvent, usually water, at subboiling temperature for a specific time. In this method, 10 g of plant material was combined with 100 mL of distilled water for 10 min. The extract obtained was then filtered using filter paper, followed by the concentration of the solution by evaporation to remove excess solvent.12 Maceration was used for the hydroalcoholic extraction. For each plant part, 10 g of powder was introduced into a bottle containing 100 mL of 70% ethanol. After 24 h of maceration, the mixture was filtered.13 The filtrate obtained was then concentrated using a vacuum rotary evaporator, maintained at a temperature of 50°C.14
Extraction yield is expressed as a percentage, resulting from the ratio between the mass of extract obtained after solvent evaporation and the total mass of powdered plant material used in the extraction process.12 The general formula for extraction yield is as follows:
R% = [(m – m0)/mt] × 100
R%: extract yield in %, m: flask mass after evaporation, m0: mass of empty flask, mt: dry mass of plant sample in g.
Determination of total phenols – The number of total phenols present in plant extracts was assessed using the Folin-Ciocalteu reagent, following an adaptation of the method described by de Singleton et al. (1999).15 Briefly, for each extract sample (5 mg/mL), 100 μL was combined in a test tube with 1 mL distilled water and 0.5 mL Folin-Ciocalteu reagent (diluted 10-fold). After 5 min, 1.5 mL sodium carbonate solution (Na2CO3) was added to the reaction medium. The resulting mixture was incubated at room temperature in the dark for 2 h, and absorbance was measured at 765 nm against a blank.16 Each determination was carried out in triplicate. The concentration of phenolic compounds in the extracts is expressed as milligram equivalent of gallic acid per gram of dry matter (mg EGA/g extract).17
Determination of flavonoids – To quantify flavonoids in C. villosa extracts, we used the aluminum trichloride method.18 500 μL of each extract to be analyzed was added to 1500 μL of 95% ethanol, 100 μL of 10% (w/v) AlCl3, 100 μL of 1M sodium acetate, and 2.8 mL of distilled water. After vigorous shaking, the mixture was incubated in the dark at room temperature for 30 min, then the absorbance was measured at 415 nm using a spectrophotometer.19 Flavonoid concentrations were determined based on a calibration curve established with quercetin as reference and are expressed in milligrams of quercetin equivalent per gram of plant dry weight (mg QE/g extract).
Determination of condensed tannins – Condensed tannins were quantified using the acid vanillin method described by Martin L.20 A quantity of 200 μL of each extract was added to 1000 μL of 4% vanillin solution; the resulting mixture was vigorously shaken and left to react at 30oC in the dark for 20 min. Absorbance was measured at a wavelength of 550 nm against a blank consisting of a mixture of ethanol (37%) and HCl (8%) using a spectrophotometer. Tests were carried out in triplicate for each sample. Catechol was used as a reference standard to establish the calibration curve and quantify condensed tannin content, expressed as milligrams of catechol equivalent per gram of dry plant matter.21
Evaluation of the antioxidant activity of the extracts in vitro – Various techniques are used to assess the antioxidant activity of extracts, most of them based on color changes or discoloration of a reagent in the reaction medium. In our research, three distinct chemical tests were used, namely: determination of total antioxidant capacity (TAC), the iron reduction test (FRAP), and finally DPPH free radical scavenging.
Scavenging the DPPH free radical – The antioxidant activity of C. villosa extracts was assessed using the DPPH assay, according to the protocol described by Mensor et al.22 DPPH (2,2-diphenyl-1-picrylhydrazyl) is a violet free radical that turns yellow when neutralized by an antioxidant. Assessment of antioxidant activity is based on the sample’s ability to neutralize this radical, which is reflected in a decrease in absorbance at a specific wavelength. The protocol used to measure the free radical scavenging activity of C. villosa extracts was detailed by Changqing Wu et al. in 2005.23 Our plant extracts and the standard were dissolved in ethanolic solution and diluted to various concentrations (0–1 mg/mL). For each sample, 900 μL of DPPH hydroalcoholic solution (0.2 mM) was mixed with 100 μL of each plant extract dilution. The reaction mixture was incubated in the dark at room temperature for 30 min. Absorbance was then measured at 517 nm against a control consisting of 900 μL DPPH solution and 100 μL ethanol. The samples, controls, standard (ascorbic acid), and blank were prepared under the same operating conditions.
Absorbance decay was measured using a UV-visible spectrophotometer. Percentage inhibition of antioxidant activity (%) was calculated according to the following equation:
% Antioxidant activity = [(control absorbance − sample absorbance) / control absorbance] × 100
IC50 values, representing the concentration required to inhibit 50% of DPPH radical activity, were determined graphically by linear regression.
Ferric reducing antioxidant power “FRAP” – Determination of the reducing power of iron was based on the method described by Benzie and Strain.24 The FRAP is based on the ability of a sample to reduce iron (III) to iron (II) in the reaction medium via an electron transfer process. The tripyridyltriazine iron complex produces an intense blue coloration measured at 593 nm by a spectrophotometer. For this study, a dilution range from 1 to 0.001 mg/mL was prepared for all extracts and the standard (ascorbic acid). The ethanol-diluted extracts were mixed with 200 μL phosphate buffer (0.2 M, pH 6.6), 200 μL potassium ferricyanide (1% K3Fe (CN)6) and 150 μL extract. The mixture was stirred and incubated at 50°C for 20 min. Next, 200 μL of trichloroacetic acid (10% TCA) was added to stop the reaction, and the mixture was centrifuged for 10 min at 3000 rpm. To the resulting solution, 600 μL of distilled water and 120 μL of ferric chloride (0.1% FeCl3) were added. A blank was prepared under the same conditions, with no sample added. Absorbance was measured at 593 nm against this blank. Ascorbic acid was used as a reference standard. The EC50 value, defined as the antioxidant concentration required to inhibit 50% of the activity, was calculated graphically.25
Determination of total antioxidant capacity TAC – Evaluation of the total antioxidant activity of the extracts was carried out using the phosphomolybdenum method, according to the protocol detailed by de Prieto, Pineda and Aguilar (1999).26 This method relies on the ability of antioxidant compounds to reduce molybdenum (VI) ions to molybdenum (V) in an acid medium, thus forming a dark green complex. For each sample, 100 μL of extract at different concentrations were mixed with 1000 μL of a reagent containing H2SO4 (0.6 M), Na2PO4 (28 mM) and ammonium molybdate (4 mM). Tubes containing this reaction mixture were hermetically sealed and incubated at 95oC for 90 min. After cooling to room temperature, absorbance was measured at a wavelength of approx. 695 nm using a spectrophotometer, in comparison with a blank prepared under the same conditions. Total antioxidant capacity was expressed in milligrams of ascorbic acid equivalent per gram of extract (mg EAA/g EX).
Determination of the antimicrobial activity – To assess the antimicrobial activity of our extracts, five microbial strains were selected; Candida albicans, Staphylococcus aureus, Dickey asolani, Pectobacterium brasiliensis, and Pectobacterium wasabiae, all isolated at the Microbial Biotechnology Laboratory of the Dhar El Mahraz Faculty of Sciences in Fez. After incubation for 24 h, the bacterial strains were prepared in suspension; 1 to 2 well-isolated, identical colonies were inoculated into 3 mL of sterile physiological water using a platinum loop. After vortex homogenization, the turbidity of the suspensions was adjusted to achieve a final concentration equivalent to a McFarland standard of 0.5, corresponding to 1.2 × 108 CFU/mL for bacteria (D.O = 0.08 to 0.1/λ = 625 nm) and 1.5 × 106 CFU/mL for C. albicans (D.O = 0.12 to 0.15/λ = 530 nm).27 For antimicrobial activity tests, a 20 μL volume of the tested microorganism suspension is withdrawn with a sterile micropipette, then deposited and spread on the surface of the Petri dish. A distilled swab was then used to make tight striations across the entire agar surface.28 Disks impregnated with antibiotics (chloramphenicol) and DMSO (20 μL per disk) were also used as controls to assess the sensitivity of the strains tested. After a 15 min rest, Petri dishes were incubated at 37oC for 24 h for bacteria and at 30oC for 48 h for yeasts. The diameters of the zones of inhibition around the discs were measured using a ruler graduated in millimeters. The sensitivity of strains to the extracts studied was classified according to their inhibition diameter (ID). All experiments were carried out in triplicates and results are presented with the mean standard deviation.29
Results and Discussion
In this study on C. villosa Link, the yields of aqueous and hydroalcoholic extracts from different parts of the plant were assessed by maceration and decoction, measuring the weight of dry plant material. The extracts obtained presented generally pasty and sometimes viscous textures, varying in color from brown to green. Extraction yields were calculated for each sample, and the results obtained are shown in Fig. 1. We observe significant variations between plant parts. This difference can be attributed to the diversity of their physicochemical composition, as indicated by Shah et al. (2017).30 Notably, hydroalcoholic extracts from flowers showed the highest yields, reaching 26%, closely followed by those from stems at 25%, compared to aqueous extracts which showed more modest yields, notably 19% for flowers. A study conducted by Abdelkarim Guaâdaoui and colleagues in 2016 revealed that hydroalcoholic extracts of Calicotome villosa (Poiret) Link showed higher yields than aqueous extracts. The highest yields were observed in seeds (13.66%) and roots (14.95%), while aqueous extracts showed the highest yield in leaves (1.5%).31 The variability of extraction yields between different plant parts and solvent types highlights the importance of considering several factors, such as plant species, harvesting conditions, solvent type, and extraction methods, when evaluating plant extracts for various applications.
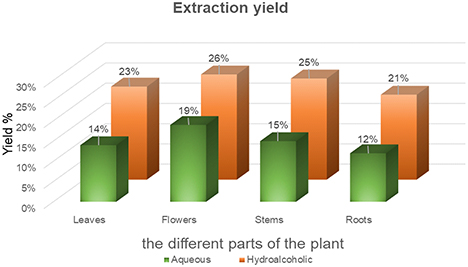
Extraction yields; Aqueous extracts were obtained by decoction; Hydroalcoholic extracts were obtained by maceration.
The results of polyphenol content are expressed as milligrams of gallic acid equivalent per gram of extract (mg EGA/g extract), calculated using a calibration curve for gallic acid based on the equation y = 0.3634x + 0.0505, with a coefficient of determination R² of 0.9988. The results of our study indicate that all parts of the plant subjected to both extraction techniques show a high concentration of polyphenols. The data show that the hydroalcoholic fraction of leaves has the highest total phenol content, reaching around 35.21 mg GAE/g extract, followed by stem extracts with a value of 28.05 mg GAE/g extract. By contrast, the aqueous fraction shows a high concentration of polyphenols in flower extracts (29.43 mg EGA/g extract). The findings of this study are in line with those reported by Murat Turan and Ramazan Mammadov (2020),32 who showed that flower extracts have higher total phenol concentrations than stem extracts. Their research revealed respective values of 159.47 ± 0.33 mg GAE/g extract for flowers and 109.67 ± 0.26 mg GAE/g extract for stems. A comparison of these results with our own suggests a higher concentration of phenolic compounds in C. villosa leaf extracts, probably attributable to the solvent used and the part of the plant selected for extraction. Previous research by Radia Cherfia et al. (2017)33 demonstrated that the ethyl acetate fraction from leaves had the highest concentration of phenols (107.75 ± 0.41 mg GAE/g). The n-butanol fractions extracted from flowers and leaves also showed significant concentrations, with values of 96.06 ± 2.72 mg GAE/g and 81.45 ± 0.60 mg GAE/g respectively. By comparison, the ethyl acetate fraction of flowers revealed a value of 64.24 ± 1.81 mg GAE/g. Nefzi et al. compared the contents of total polyphenols, flavonoids and tannins of ethanolic extracts from the leaves of different plants including C. villosa. Their results showed that, compared to the other plants studied, C. villosa has a fairly low content of total polyphenols (32 mg GAE/g) and flavonoids (4 mg of catechin equivalent per gram of dry weight).On the other hand, it had the highest tannin content (25 mg CE/g).34
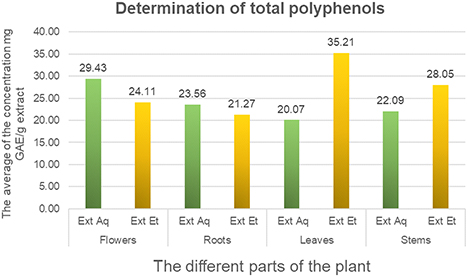
Total polyphenols content in aqueous and hydroalcoholic extracts of different parts of C. villosa. The results presented are expressed as milligrams of gallic acid equivalent per gram of extract (mg EGA/g extract), calculated using a calibration curve for gallic acid based on the equation y = 0.3634x + 0.0505, with a coefficient of determination R² of 0.9988.
Work carried out by Boughalleb et al. on monitoring the phenolic composition of C. villosa seeds during storage showed that C. villosa seeds are rich in phenolic compounds with values ranging from 34.6 to 45.1 mg GAE/g DW.35 these values are similar to those of other leguminous seeds such Lathyrus sativus (37.5 mg GAE/g DW) and Mucuna pruriens (33.04 mg GAE/g DW).36
Flavonoids were evaluated using the aluminum trichloride (AlCl3) method, with significant variations between varieties. Each extract was reported in milligrams equivalent of quercetin per gram of plant dry matter (mg QE /g extract), according to a linear equation y = 1.684x + 0.2128 with a correlation coefficient R² = 0.9967. The total amount of flavonoids in the leaves, stems, roots and flowers of both the hydroalcoholic and aqueous fractions of C. villosa is shown in Fig. 3. Our results indicate that the highest values were obtained in the hydroalcoholic and aqueous fractions of leaves (58.68 ± 0.10 and 51.29 ± 0.001 mgQE/g extract), with a significant predominance of the hydroalcoholic fraction in leaves and stems.
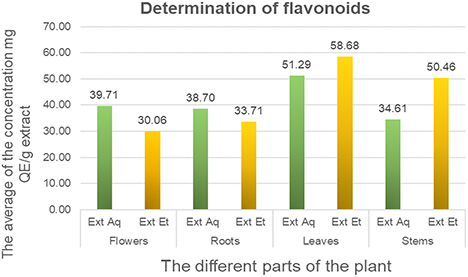
Flavonoids content in aqueous and hydroalcoholic extracts of different parts of C. villosa. Each extract was reported in milligrams equivalent of quercetin per gram of plant dry matter (mg QE/g extract), according to a linear equation y = 1.684x + 0.2128 with a correlation coefficient R² = 0.9967.
Furthermore, our results agree with those of Radia et al. showing high values in the ethyl acetate fraction of leaves (20.87 ± 0.13 mg QE/g extract), followed by the n-butanol fraction of leaves (17.03 ± 0.06 mgQE/g extract), and a lower concentration in the n-butanol fraction of flowers (8.19 ± 0.44 mg QE/g extract).33 The results of Elkhamlichi et al. confirm that our findings on extracts from pods and seeds of Calycotome villosa subsp. Intermedia indicate a higher content in ethanolic extracts than in ethyl acetate extracts. Values ranged from 5.02 ± 0.03 to 66.45 ± 0.01 mg QE/g extract.37 In contradiction with our results, Previous work by Turan et al. revealed different levels. They found higher concentrations in flower extracts than in stem extracts, with respective values of 66.21 ± 0.09 mgQE/g and 18.41 ± 0.02 mg QE/g extract for hydroalcoholic extracts, while our results give the following values (504.56 ± 0.02 mg QE/g extract for stems and 397.1 ± 0.04 mg/gEQ for flowers).32 It is difficult to compare our results directly with those in the bibliography, as many factors can influence the qualitative and quantitative composition of flavonoids like the part of the plant used, the harvesting period, and the solvent used for extraction.
Quantification of tannins in extracts was carried out using a calibration curve based on catechin as the standard. Results were calculated from the linear regression equation of this curve and are expressed inmg EC/g dry extract. Analysis of the results for tannin content in extracts reveals significant variations between different plant organs. In general, roots show the highest concentrations, followed by flowers and leaves, while stems show the lowest concentrations. According to the data in Fig. 4, condensed tannins range from 21.30 ± 2.36 to 8.11 ± 1.22 mg EC/g dry extract for the hydroalcoholic fraction, and from 10.54 ± 0.41 to 3.45 ± 0.71 mg EC/g dry extract for the aqueous fraction. These results indicate that the hydroalcoholic fraction contains the highest concentrations of tannins, with a maximum value of 21.30 mg EC/g dry extract in roots, while the aqueous fractions show more moderate levels. Nefzi et al. showed that ethanolic extracts of C. villosa leaves have high tannin contents exceeding those of Pistacia lentiscus, Rosmarinus officinalis and Phillyrea latifolia, measured at 17.36, 8.5 and 10 mg CE/gDW respectively.34 Phytochemical analysis, recently carried out by Aljabri et al., showed the presence of alkaloids, carbohydrates, saponins, phytosterols, phenols and tannins in the methanolic extracts of C. villosa. These active constituents showed antidepressant, muscle relaxant, CNS depressant, and antipsychotic-like activity.38 These results highlight the therapeutic potential of C. villosa and encourage future research into its specific medicinal applications. This variation of tannins content can be explained by the fact that the quantification of condensed tannins depends on their chemical nature, the solvent used for extraction and the operating conditions.39
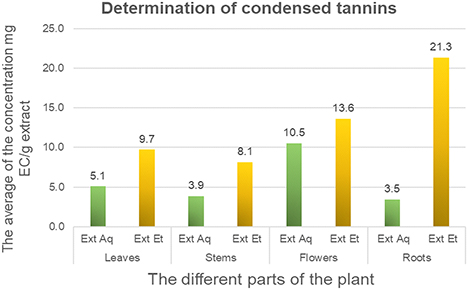
Tannins content in aqueous and hydroalcoholic extracts of different parts of C. villosa. Quantification of tannins in extracts was carried out using a calibration curve based on catechin as the standard.
The antioxidant activities of the hydroalcoholic and aqueous extracts of C. villosa were carried out using three assays; The phosphomolybdenum method, the DPPH assay and the Fe3+ reduction assay to evaluate the reducing power. The antioxidant capacity of the different extracts is determined by the IC50. This is the concentration of extract necessary to reduce 50% of the DPPH radical. The IC50 and the antioxidant activity of the tested extracts are inversely proportional. The IC50 values are represented in Fig. 5. Comparing the IC50 of the different tested extracts of C. villosa with that of ascorbic acid, we notice that the antiradical activity of all our extracts is lower than that of ascorbic acid whose IC50 is 0.001 mg/mL. The hydroalcoholic extract of the leaves (0.05 mg/mL) shows the higher antioxidant power followed by the aqueous extract of the same part of plant and the hydroalcoholic extract of flowers. We also notice, for the rest of the extracts, a similar activity between them. Results obtained by Chikhi et al. (2014),40 concerning two C. villosa leaf extracts, namely the essential oil and the ethanolic extract, demonstrated significant antioxidant activity. The essential oil showed a radical scavenging activity of 60%, while the ethanolic extract showed the highest activity with 96% at a concentration of 200 μg/mL, compared to ascorbic acid which showed an effect of 98.61% at the same concentration. Turan et al. examined antioxidant activity by the DPPH method, showing that stems and flowers presented the highest activity with IC50 values of 70.09 ± 0.10 mg/mL and 86.34 ± 0.16 mg/mL respectively.32 Cherfia et al. demonstrated that fractions extracted from C. spinosa leaves generally possessed higher antioxidant activity than those extracted from flowers. They observed IC50 values of 45.25 ± 1.8 mg/mL (ethyl acetate) and 52.80 ± 2.05 mg/mL (n-butanol) for leaves, while those for flowers were 63.3 ± 0.12 mg/mL (ethyl acetate) and 53.95 ± 0.19 mg/mL (n-butanol) respectively.33 These results are in line with our observations. It is likely that the activity of extracts is attributed to their richness in phenolic compounds. Numerous studies have shown a correlation between the total polyphenol content of plants and their antioxidant capacities.41 According to De Pooter et al. antioxidant molecules such as ascorbic acid, flavonoids, and tannins reduce and decolorize DPPH due to their ability to donate hydrogen.42
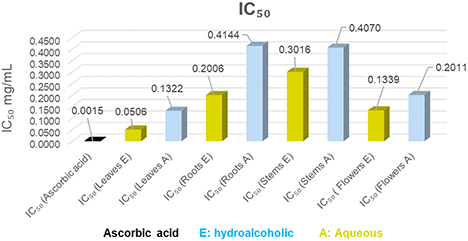
The IC50 of the extracts of different parts of Calicotome villosa and ascorbic acid. IC50 is the concentration of extract necessary to reduce 50% of the DPPH radical.
The FRAP method is recognized for its simplicity, rapidity and reproducibility, making it applicable to both organic and aqueous extracts, as well as plant tissues and plasmas.24,43 In our study, we used the FRAP method to test various plant extracts. The results obtained enabled us to plot curves for each extract, revealing a dependence of iron reduction capacity on sample concentration.44 From Fig. 6, we observed that extracts from different parts of C. villosa, notably those from the leaves and stems, showed notable reducing capacity. The aqueous extract of leaves and stems showed a high reducing capacity compared to the methanolic extract, slightly lower than that of ascorbic acid, especially at the highest concentrations. At the lowest concentrations, stem and leaf extracts demonstrated a higher iron-reducing capacity than ascorbic acid. These results indicate that the extracts probably contain phenolic or other compounds capable of donating electrons or hydrogen.45,46 In their study, Elkhamlichi et al. found that ethyl acetate extracts of Calycotome villosa subsp. intermedia seeds exhibit high reducing power compared with the methanolic extract. At a concentration of 0.32 mg/mL, these extracts showed a higher reducing capacity than ascorbic acid. In contrast, the reducing power of the methanolic and ethyl acetate extracts of the pods was lower than that of ascorbic acid.37 In conclusion, C. villosa extracts possess an interesting capacity to reduce iron, suggesting a high antioxidant potential due to their richness in phenolic compounds.47 The reducing power of plant extracts is often considered a significant indicator of their potential antioxidant activity, as other previous studies have also shown.48–50
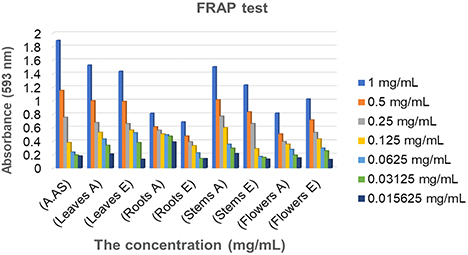
Absorbance of different concentrations of the aqueous and hydroalcoholic extracts studied by FRAP method; Absorbance is proportional to antioxidant capacity.
The total antioxidant capacity is expressed in milligrams equivalent of ascorbic acid per gram of dry extract (mg EAA/g extract). The total antioxidant capacity levels of the extracts were obtained from a calibration curve of ascorbic acid. The results show that all the extracts exhibit different antioxidant activities with high values in the hydroalcoholic extracts compared to the aqueous ones. The extract from the underground part of the studied plant has the best total antioxidant capacity of the order of 0.34 ± 0.07 mg EAA/ g extract for the hydroalcoholic fraction and 0.29 ± 0.02 mg EAA/g extract of the aqueous extract. The hydroalcoholic extract of leaves shows also an interesting activity. According to Nefzi et al. C. villosa exhibited the highest antioxidant capacity with values measured at 15.33 ± 0.39 mg GAE/gDW, compared to Rosmarinus officinalis which showed a capacity of 12.10 ± 0.53 mg GAE/gDW.34 In a study conducted by Elkhamlichi et al.37 it was observed that the ethyl acetate extract of the seeds exhibited significant total antioxidant capacity, equivalent to 157.6 mg/g ascorbic acid, at a higher concentration (100 μg/mL). By contrast, crude pod extracts showed the lowest antioxidant activity. This antioxidant power observed in these extracts could be due mainly to their richness in phenolic compounds, but also to the chemical structures of the bioactive molecules.
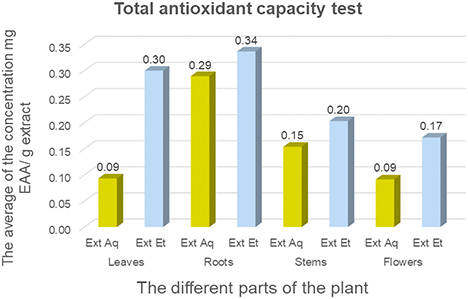
The antioxidant activity of different parts of C. villosa extracts by Total Antioxidant Capacity (TAC) method using phosphomolybdenum.
In order to evaluate the antimicrobial activity of our extracts, we used the disc diffusion method. It is a qualitative technique based on the measurement of the apparent inhibition diameter around the disks loaded with plant extracts. The qualitative evaluation of the antimicrobial activity was performed on four fractions of C. villosa (flowers, leaves, stems, and roots). Different concentrations (10 and 50 mg/mL) of hydroalcoholic and aqueous extracts of C. villosa were prepared using DMSO. These extracts were evaluated for their antimicrobial activities against the tested microorganisms. We report in Table 1 the diameters of the inhibition zones (expressed in mm) of the different extracts of C. villosa against five microbial strains.51
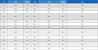
Antimicrobial activity of aqueous and hydroalcoholic extracts of different parts of C. villosa expressed by diameters of the inhibition zones
According to the values recorded in Table 1, the extracts show moderate antibacterial activity against all the strains studied (diameter of the inhibition zones between 8.6 and 21 mm) with the exception of C. albicans for which no activity was observed. Hydroalcoholic extracts of C. villosa leaves, roots and flowers demonstrated significant inhibition of the growth of Gram-positive S. aureus bacteria. The zones of inhibition observed were: 16.5 ± 0.07 mm (FL/H), 15 ± 0 mm (R/H), and 11.5 ± 0.07 mm (L/H), all recorded at a concentration of 10 mg/mL, lower than that used for other bacteria (50 mg/mL).
This difference could be due to structural variations between Gram-positive and Gram-negative bacteria. It is also worth noting that the hydroalcoholic extracts of the various plant parts studied showed a remarkable effect on the S. aureus strain, while the aqueous extracts had no effect at all. Although the aqueous extract showed the lowest efficacy against this bacterium, the hydroalcoholic extracts were more potent. This may be due to the fact that most of the compounds shown to be active against antimicrobial strains are not water-soluble.40,8 Previous studies such as that by Alhage et al. have also highlighted that C. villosa stem extracts obtained with solvents such as dichloromethane and methanol were more effective than the aqueous extract.8 At a concentration of 1mg/mL, the methanol extract showed significant zones of inhibition against various strains, including 16 mm for K. pneumoniae and 17 mm for C. albicans, confirming the antimicrobial activity of these extracts. Thus Chikhi et al.40 showed the efficacy of hydroalcoholic extracts of the aerial parts of the C. villosa plant against S. aureus with a zone of inhibition of 10 mm. From the concentration of 50 mg/mL, the bacteria P. wasabiae and P. brasiliensis (Gram-) register a remarkable sensitivity on both aqueous and hydroalcoholic extracts for the different parts of the plant with a higher diameter of 21 mm in the (Hydroalcoholic Roots extract), followed by 20.60 mm (Aqueous Stem extract) and 20.30 mm (Aqueous Leaves extract) in P. brasiliensis. On the other hand, the different fractions of flowers, stems, roots and blossoms (hydroalcoholic and aqueous) revealed significant activity against P. brasiliensis (21 ± 0.1 and 20.3 ± 0.15 and 20.6 ± 0.12 mm, successively), however a lesser antimicrobial potential against P. wasabiae (11 ± 0.1 and 8.6 ± 0.153, respectively) was observed.
In addition, studies by Loy et al. showed that the essential oil and methanol crude extract of C. villosa leaves were also highly active against several Grampositive bacteria, including S. aureus ATCC 25923 (20 and 10 mm respectively), Bacillus lentus B 60 (10 and 11 mm in that order), Escherichia coli ATCC 25922 (15 and 10 mm) and Klebsiella. pneumoniae 52 (12 and 10 mm).2 Results from Cherfia et al. confirmed significant antibacterial activity of C. villosa leaf fractions against Gram-positive and Gram-negative bacteria, with zones of inhibition (7 ± 0.41 to 22 ± 0.06 mm) and 7 ± 0.76 to 13 ± 0.12 mm for flower fractions. These values show that leaf fractions are more active than flower fractions.33
The results of Dickey asolani (Gram-) show a significant sensitivity against the hydroalcoholic extracts of the different parts of the plant with inhibition zones of 18 ± 0.1 mm (Roots) and 11 ± 0.1 mm (stems) and 18 ± 0.27mm (leaves) and 9.67 ± 0.06 mm (Flowers) respectively, on the other hand, this strain presented a resistance against the aqueous extract. Nevertheless, C. albicans is yeast resistant to all tested extracts of our plant. Our results confirm the work of Chikhi et al.40 who found no susceptibility of C. albicans against hydroalcoholic extracts of the aerial parts of the same plant. C. albicans. E. coli and S. aureus are recognized as food contaminants.52
In general, Gram-positive bacteria were found to be more sensitive than Gram-negative bacteria. This increased sensitivity is attributed to the presence of hydrophobic lipopolysaccharides in the outer membrane of Gramnegative bacteria. This observation is consistent with previous studies which have highlighted that differences in sensitivity are due to variation in the chemical composition and structure of the cell wall between these two types of micro-organisms. Thus, the complexity of the cell wall structure of Gram-negative bacteria acts as a barrier that limits the penetration of antimicrobial agents.53,54
In conclusion, the present study has enabled us to highlight two major aspects: the quantification of polyphenolic compounds and the evaluation of the antioxidant and antimicrobial activity of hydroalcoholic and aqueous extracts from various C. villosa organs. In the light of our investigations, several main conclusions can be drawn; Firstly, assessment of the content of total polyphenols, flavonoids and condensed tannins revealed the presence of moderately significant quantities of polyphenolic compounds across the various plant organs.
A study of the antioxidant activity of C. villosa extracts, carried out using the iron reduction method and the DPPH free radical scavenging method, revealed moderate antioxidant activity for both hydroalcoholic and aqueous extracts. This antioxidant capacity seems to be attributed to the presence of phenolic compounds in the extracts. Finally, with regard to antibacterial activity against pathogenic and multi-resistant bacterial strains, the hydroalcoholic fractions from the various plant parts demonstrated significant antibacterial efficacy, with the exception of C. albicans, for which no activity was observed. Inhibition of bacterial growth varied according to bacterial species, the concentration of extracts tested, and the culture medium used. This study underlines the potential of C. villosa as a valuable source of bioactive compounds, highlighting its diverse therapeutic applications particularly in antioxidant and antimicrobial treatments.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Kaneria, M.; Kanani, B.; Chanda, S. Asian Pac. J. Trop. Biomed. 2012, 2, 195–202.
[https://doi.org/10.1016/S2221-1691(12)60041-0]

-
Loy, G.; Cottiglia, F.; Garau, D.; Deidda, D.; Pompei, R.; Bonsignore, L. Farmaco. 2001, 56, 433–436.
[https://doi.org/10.1016/S0014-827X(01)01056-4]

-
Palu, D. S.; Paol, M.; Casabianca, H.; Casanova, J.; Bighelli, A. Molecules 2020, 25, 3467.
[https://doi.org/10.3390/molecules25153467]

- Greuter, W.; Burdet, H. M.; Long, G. MED CHECKLIST: Acritical inventory of vascular plants of the circum-mediterranean countries - Volume 1 (Pteridophyta); Conservatory and Botanical Garden of the City of Geneva, OPTIMA; Switzerland, 1984, p 330.
-
Hartwell, J. L. Lloydia 1969, 32, 78–107.
[https://doi.org/10.1515/crll.1969.235.78]

- Lentini, F.; Aleo, M.; Amenta, R. Acta phytoterapeutica 1997, 4, 88–94.
-
El Antri, A.; Lachkar, N.; El Hajjaji, H.; Gaamoussi, F.; Lyoussi, B.; El Bali, B.; Morel, N.; Allouchi, H.; Lachkar, M. Arab. J. Chem. 2010, 3, 173–178.
[https://doi.org/10.1016/j.arabjc.2010.04.006]

-
Alhage, J.; Elbitar, H.; Taha, S.; Guegan, J.-P.; Dassouki, Z.; Vives T.; Benvegnu, T. Molecules 2018, 23, 851.
[https://doi.org/10.3390/molecules23040851]

-
Valko, M.; Rhodes, C. J.; Moncol, J.; Izakovic, M.; Mazur, M. Chem. Biol. Interact. 2006, 160, 1–40.
[https://doi.org/10.1016/j.cbi.2005.12.009]

-
Rajkumar, V.; Guha, G.; Kumar, R. A. Food Chem. Toxicol. 2011, 49, 363–369.
[https://doi.org/10.1016/j.fct.2010.11.009]

-
Karaman, I.; Sahin, F.; Güllüce, M.; Ögütçü, H.; Sengül, M.; Adıgüzel, A. J. Ethnopharmacol. 2003, 85, 231–235.
[https://doi.org/10.1016/S0378-8741(03)00006-0]

- Bohui, P. S. G.; Adima, A. A.; Niamké, F. B.; N’Guessan, J. D. J. Soc. Ouest-Afr. Chim. 2018, 46, 50–58.
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. J. Food Drug Anal. 2002, 10, 178–182.
-
Turan, M.; Mammadov, R. PP 2018, 9, 100–116.
[https://doi.org/10.4236/pp.2018.94008]

-
Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Methods Enzymol. 1999, 299, 152–178.
[https://doi.org/10.1016/S0076-6879(99)99017-1]

-
Spanos, G. A.; Wrolstad, R. E. J. Agric. Food Chem. 1990, 38, 1565–1571.
[https://doi.org/10.1021/jf00097a030]

- Boizot, N.; Charpentier, J.-P. INRA 2006, 79–82.
-
Horszwald, A.; Andlauer, W. J. Berry Res. 2011, 1, 181–199.
[https://doi.org/10.3233/JBR-2011-020]

-
Moreno, M. I.; Isla, M. I.; Sampietro, A. R.; Vattuone, M. A. J. Ethnopharmacol. 2000, 71, 109–114.
[https://doi.org/10.1016/S0378-8741(99)00189-0]

-
Price, M. L.; Scoyoc, S. V.; Butler, L. G. J. Agric. Food Chem. 1978, 20, 1214–1218.
[https://doi.org/10.1021/jf60219a031]

-
Kouamé, T. K.; Siaka, S.; Kassi, A. B. B.; Soro, Y. Int. J. Biol. Chem. Sci. 2021, 15, 97–105.
[https://doi.org/10.4314/ijbcs.v15i1.9]

-
Mensor, L. L.; Menezes, F. S.; Leitao, G. G.; Reis, A. S.; dos Santos, T. C.; Coube, C. S.; Leitao, S. G. Phytother. Res. 2001, 15, 127–130.
[https://doi.org/10.1002/ptr.687]

-
Wu, C.; Chen, F.; Wang, X.; Kim, H.-J.; He, G.-Q.; Haley-Zitlin, V.; Huang, G. Food Chem. 2006, 96, 220–227.
[https://doi.org/10.1016/j.foodchem.2005.02.024]

-
Benzie, I. F.; Straint, J. J. Anal. Biochem. 1996, 239, 70–76.
[https://doi.org/10.1006/abio.1996.0292]

- Addab, N.; Fetni, S.; Hamlaoui, F.; Zerguine, A.; Mahloul, K. J. Fac. Mad. Or. 2020, 579–586.
-
Prieto, P.; Pineda, M.; Aguilar, M. Anal. Biochem. 1999, 269, 337–341.
[https://doi.org/10.1006/abio.1999.4019]

-
Pfaller, M. A.; Messer, S. A.; Karlsson, A.; bolmstrom, A. J. Clinl. Microbiol. 1998, 36, 2586–2589.
[https://doi.org/10.1128/JCM.36.9.2586-2589.1998]

- Biyiti, L. F.; Meko’o, D. J. L.; Tamzc, V.; Amvam Zollo, P. H. Pharm. Méd. Trad. Afr. 2004, 13, 11–20.
-
Espinel-Ingroff, A. Clin. Microbiol. Newsl. 2007, 29, 97–100.
[https://doi.org/10.1016/j.clinmicnews.2007.06.001]

-
Van loy, M. D.; Riley, W. J.; Daisey, J. M.; Nazaroff, W. W. Environ. Sci. Technol. 2001, 35, 560–567.
[https://doi.org/10.1021/es001372a]

- Guaâdaoui, A.; El-alami, I.; Abid, M.; Boukhatem, N.; Lechkar, M.; Hamal, A. IJGHC 2016, 5, 93–111.
-
Turan, M.; Mammadov, R. Pharm. Chem. J. 2020, 54, 478–483.
[https://doi.org/10.1007/s11094-020-02225-8]

- Cherfia, R.; Kara Ali, M.; Talhi, I.; Benaissa, A.; Chaouche, N. K. J. Pharmacognosy Phytother. 2017, 9, 185–196.
-
Nefzi, K.; Ben Jemaa, M.; Baraket, M.; Dakhlaoui, S.; Msaada, K.; Nasr, Z. Appl. Sci. 2022, 12, 5038.
[https://doi.org/10.3390/app12105038]

-
Boughalleb, F.; Mahmoudi, M.; Abdellaoui, R.; Yahia, B.; Zaidi, S.; Nasri, N. J. Food Biochem. 2019, 44, 1–13.
[https://doi.org/10.1111/jfbc.13093]

-
Wang, T.; Tang, X. C. Eur. J. Pharmacol. 1998, 349, 137–142.
[https://doi.org/10.1016/S0014-2999(98)00199-X]

- Elkhamlichi, A.; El Hajaji, H.; Faraj, H.; Alami, A.; El Bali, B.; Lachkar, M. J. Appl. Pharm. Sci. 2017, 7, 192–198.
- Aljabri, S. O.; Aburawi, S. M.; Alkayed, F. W.; Alrbige, N. A.; Akordi, N. E. South Asian Res. J. Pharm. Sci. 2023, 5, 1–17.
- Ali-Rachedi, F.; Meraghni, S.; Touaibia, N.; Sabrina M. Bull. Soc. R. Sci. Liège. 2018, 87, 16–19.
-
Chikhi, I.; Allali, H.; Bechlaghem, K.; Fekih, N.; Muselli, A.; Djabou, N.; Dib, M. E. A.; Tabti, B.; Halla, N.; Costa, J. Asian Pac. J. Trop. Dis. 2014, 4, 356–362.
[https://doi.org/10.1016/S2222-1808(14)60587-9]

-
Lamien-Meda, A.; Lamien, C. E.; Compaoré, M. M. Y.; Meda, R. N. T.; Kiendrebeogo, M.; Zeba, B.; Millogo, J. F.; Nacoulma, O. G. Molecules 2008, 13, 581–594.
[https://doi.org/10.3390/molecules13030581]

- Talbi, H.; Boumaza, A.; El-mostafa, K.; Talbi, J.; Hilali, A. Mater. Environ. Sci. 2015, 6, 1111–1117.
-
Lia, H. B.; Wonga, C. C.; Chenga, K. W.; Chen, F. LWT 2008, 41, 385–390.
[https://doi.org/10.1016/j.lwt.2007.03.011]

-
Bentabet, N.; Boucherit-Otmani, Z.; Boucherit, K. Pharmacognosie. 2014, 12 364–371.
[https://doi.org/10.1007/s10298-014-0834-x]

-
Pyo, Y.-H.; Lee, T.-C.; Logendra, L.; Rosen, R. T Food Chem. 2004, 85, 19–26.
[https://doi.org/10.1016/S0308-8146(03)00294-2]

- Muflihah, Y. M.; Gollavelli, G.; Ling, Y.-C. Antioxidants 2004, 10, 1–15.
-
Siddhuraju, P.; Becker, K. Food Chem. 2007, 101, 10–19.
[https://doi.org/10.1016/j.foodchem.2006.01.004]

-
Jeong, S. M.; Kim, S. Y.; Kim, D. R.; JO, S. C.; Nam, K. C.; Ahn, D. U.; Lee, S. C. J. Agric. Food Chem. 2004, 52, 3389–3393.
[https://doi.org/10.1021/jf049899k]

-
Kumaran, A.; Karunakaran, R. J. LWT 2007, 40, 344–352.
[https://doi.org/10.1016/j.lwt.2005.09.011]

-
Ghedadba, N.; Hambaba, L.; Ayachi, A.; Aberkane, M. C.; Bousselsela, H.; Oueld-Mokhtar, S. M. Phytothérapie 2017, 13, 118–129.
[https://doi.org/10.1007/s10298-015-0944-4]

-
Ponce, A. G.; Fritz, R.; del Valle, C.; Roura, S. I. LWT 2003, 36, 679–684.
[https://doi.org/10.1016/S0023-6438(03)00088-4]

-
Al-Hadhrami, R. M. S.; Hossain, M. A. Egypt. J. basic Appl. Sci. 2016, 3, 329–334.
[https://doi.org/10.1016/j.ejbas.2016.08.001]

-
Alzoreky, N. S.; Nakahara, K. Int. J. Food Microbiol.2003, 80, 223–230.
[https://doi.org/10.1016/S0168-1605(02)00169-1]

-
Cowan, M. M. Clin. Microbiol. Rev. 1999, 12, 564–582.
[https://doi.org/10.1128/CMR.12.4.564]
