The Therapeutic Effects of Native Halophyte, Suaeda glauca Bunge and Its Constituents on Benign Prostatic Hyperplasia
Abstract
Benign prostatic hyperplasia (BPH) is one the most common urinary disorders in elderly men, occurring when the prostate, which surrounding the urethra, undergoes abnormal enlarges and obstructs urine flow. Within the prostate, testosterone is converted to dihydrotestosterone (DHT) by the lipophilic enzyme 5α- reductase. Excessive accumulation of DHT binds to androgen receptors (AR), causing the hyperproliferation of stromal and epithelial cells, which increases the size of the prostate. In the process of exploring new materials with efficacy for improving BPH from halophytes native to Korea, Suaeda glauca (Bunge) Bunge was found as a promising candidate. S. glauca is an annual plant belonging to the Amaranthaceae family. In human prostate cell lines RWPE-1 and LNCaP, the ethanolic extract of S. glauca (ESG) effectively reduced the expression levels of AR, 5α-reductase type 2 (5αR2), proliferating cell nuclear antigen (PCNA) and prostate-specific antigen (PSA) proteins, compared to finasteride, a positive control. HPLC-DAD analysis of ESG using eight compounds isolated from S. glauca identified kaempferol-3-O-β-D-glucoside as one of the major components in ESG. Among the compounds 1–8, the effects of hydroxytyrosol (8) on BPH-related proteins were most remarkable in both cell lines. These results suggest that S. glauca and its bioactive compounds may be promising novel candidates for the prevention or treatment of BPH.
Keywords:
Halophytes, Suaeda gluca, Benign prostatic hyperplasia, In vitro, LNCaP, RWPE-1Introduction
Benign prostatic hyperplasia (BPH) is a prevalent in urinary tract condition among middle-aged and older men, with an incidence that increases with age, affecting approximately 70% to 80% in those over 80 years old.1 BPH is a disease in which the size of prostate enlarges due to proliferation of stromal muscle and glandular epithelial cells within the prostatic tissue.2 BPH often causes lower urinary tract symptoms (LUTS) such as urinary leakage, nocturia, incomplete bladder emptying, and weakened urine stream.3 Among men with LUTS, 30% to 48% reported experiencing moderate or severe symptoms,4 which significantly reduce health-related quality of life and lead to complications such as urine retention, urinary tract infections, and bladder stones.5
5-Alpha reductase, an enzyme that plays a role in steroid metabolism, converts testosterone to dihydrotestosterone (DHT). DHT, with a more affinity for androgen receptor (AR) than testosterone, acts a critical role in BPH progression by promoting prostate cell growth, and 5α- reductase contributes a key role in BPH pathogenesis by promoting prostate cell proliferation.6,7 As men age, serum testosterone levels decrease, but DHT levels remain relatively high, contributing to increased androgen receptor activity.8 DHT, which binds more strongly to androgen receptors than testosterone, acts as a more potent androgen. These hormonal changes associated with aging are key contributors to prostate enlargement.9 As men age, 5α- reductases activity and the transactivation function of AR tend to increase due to an imbalance in androgen. Prostate cell proliferation and surviving are promoted by activation of AR through DHT binding. Elevated DHT levels enhance AR transactivation, which subsequently raises prostate-specific antigen (PSA) levels, an indicator of prostate health.10,11 In clinical practice, finasteride and dutasteride are main 5α-reductase inhibitors utilized to manage BPH by reducing DHT levels. Finasteride, a synthetic azasteroid, selectively reduces DHT levels by targeting the 5αR2 enzyme, especially in tissues where 5αR2 predominates.12 Conversely, dutasteride inhibits both 5αR1 and 5αR2 enzymes, resulting a more extensive suppression of DHT. Studies have shown that both drugs can lower serum DHT levels by up to 70% to 90%,13 offering significant potential in slowing BPH progression. Finasteride is effective in managing BPH, but it is associated with adverse effects such as decreased libido, gynecomastia, and the rareness of orthostatic hypotension.14 Additionally, other adverse effects of 5-alpha reductase inhibitors, including finasteride, may include dizziness, skin rash, insulin resistance, and an increased risk of metabolic conditions like non-alcoholic fatty liver disease.15,16
Suaeda glauca is an annual halophytic herb belonging to the Amaranthaceae family and is widely distributed in inland saline soils and seashore salt marsh along the coasts of Mongolia, China, Japan and Siberia, including Korea.17,18 Recent phytochemical studies on S. glauca have reported a variety of constituents including flavonoids, pentacyclic triterpenoids, steroids, phenolics and coumarins.19–22 Additionally, in biological activity of S. glauca such as antioxidant,23 anti-fibrotic,24 and anti-inflammatory25,26 have been reported. In our previous study, we isolated and identified eight compounds from the aerial parts of S. glauca and demonstrated the improvement efficacy of S. glauca extract and its compounds against hair loss,27 Based on the potent activity of the extract and compounds of S. glauca on 5αR2 in in vitro model of hair loss. This study aimed to investigate their therapeutic effects on BPH using TPactivated LNCaP and RWPE-1 cells. The changes in the expression of BPH-related proteins, 5αR2, PCNA, AR and PSA following treatment with the extract and compounds of S. glauca were measured by Western blot in two cell lines. Also, The HPLC chromatogram of ethanol extract of S. glauca (ESG) was analyzed to identify the major components contained in the extract using the compounds isolated in our previous study.27
Experimental
General experimental procedures – High-pressure liquid chromatography (HPLC; UltiMate 3000, Dionex, Thermo Scientific, Karlsruhe, Germany) consisted of a pump, temperature controller, autosampler and diode array detector. A Phenomenex Synergi 4μm Polar column (250 × 4.6 mm) was used for chromatographic separation of the ethanolic extract of S. glauca. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, dimethyl sulfoxide (DMSO), and finasteride were purchased from Sigma-Adrich (St. Louis, MO, USA). HPLC-grade water and methanol were purchased from Honeywell Burdick & Jackson (Muskegon, MI, USA). Ethanol was purchased from Daejung Chemical & Metals Co. Ltd (Gyeonggi-do, Korea). Mammalian protein extraction reagent (M-PERTM) was purchased from Thermo Scientific (Waltham, Massachusetts, USA). Protein assay dye reagent concentrate, trans-blot turbo 5x transfer buffer and enhanced chemiluminescence (ECL) were purchased from Bio Rad (California, CA, USA). Testosterone propionate (TP) was purchased from Tokyo Chemical Industry Co., Ltd (TCI; Tokyo, Japan). 5αR2, β-actin and α-tubulin were obtained from Santa Cruz Biotechnology (California, CA, USA). AR, PSA and PCNA were obtained from Cell Signaling Technology (Massachusetts, USA).
Plant material and preparation of extract – In 2018, the aerial parts of S. glauca were collected in Sinan-gun, Korea. The plant identification was confirmed by Prof. Min Hye Yang, Pusan National University, Korea. A voucher specimen (voucher number GNT-69) has been deposited at the pharmacognosy laboratory, Gyeongsang National University, Korea. The aerial parts of S. glauca were freezedried, powdered and extracted using 50% (v/v) ethanol at 70℃ for 3 h. The solvent was subsequently evaporated under reduced pressure.
HPLC analysis – The ethanolic extract of S. glauca was dissolved in 50% methanol, and the isolated compounds were dissolved with methanol and filtered through 0.45 μm PVDF filter. The HPLC system used for the analysis was an UltiMate 3000 with a Phenomenex synergi 4 μm Polar (4.6 × 250 mm). D.W. (A) and methanol (B) were used as mobile phases with a linear gradient as follows: 45–50% B for 0–25 min, 45–50% B; 25–30 min for 50–65% B. The elution was detected at 245 nm with a flow rate of 1.0 mL/min at 25℃.
Isolation of compounds – In our previous study, eight compounds (1-8) were isolated and identified from aerial parts of S. glauca.27 Brief, the aerial parts of S. glauca were extracted with 80% methanol and the partitioned by n-hexane, chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol. The EtOAc fraction was separated by various chromatography. The EtOAc fraction was separated by silica gel column chromatography with mixture of CHCl3 → MeOH → water gradient elution, to obtain 23 subfractions (E1–E23). Fraction and purification of subfractions were conducted on selected fractions using mediumpressure liquid chromatography (MPLC) with C18 reversedphase columns and HPLC on various C18 columns. Fraction E20 was further fractionation by MPLC to obtain 18 fractions (E20-1-18) from which compounds 5 and 6 were purified using HPLC (MeOH → water 50:50). In addition, fraction E7 was processed through silica gel and ODS gel chromatography, to yield compounds 1–4, 7 and 8 through HPLC under MeOH → water gradient or isocratic conditions.
Cell cultures – LNCaP (human prostatic adenocarcinoma cell line) and RWPE-1 (human prostatic epithelial cell line) were obtained from American Type Culture Collection (ATCC, Manassas, VA, United States). LNCaP cells were cultured in RPMI-1640 media (GenDEPOT, Texas, USA) containing 1% (v/v) antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin) and 10% (v/v) and Fetal Bovine Serum (FBS; GenDEPOT, Texas, USA). RWPE-1 cells were maintained in keratinocyte serum free medium (K-SFM; Gibco BRL, Grand Island, NY, USA) containing 1% (v/v) antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin), 0.05 mg/mL bovine pituitary extract and 5 ng/mL epidermal growth factor. The cells were maintained at 37℃ and 5% CO2.
Cell viability assay – Cell viability was evaluated by the MTT assay. LNCaP and RWPE-1 cells (1 × 105 cells/mL) were seeded in 96 well plate. After incubation for 24 h, cells were treated with 50% EtOH extract of S. glauca (6.25, 12.5 and 25 μg/mL) or each compound (0.1–10 μM) for 24 h. 10 μL of MTT solution (2 mg/mL) was added to each well. After 3 h incubation, the supernatant was removed and formazan crystals were dissolved with 100 μL of DMSO. Formazan was completely dissolved, absorbance was measured at 540 nm using microplate reader (Infinite M200Pro, Tecan, Switzerland). All experiments were conducted in triplicate and the percentage of cell viability was determined using non-treated control cells.
Western blotting – LNCaP and RWPE-1 cells were seeded at a density of 4 × 105 cells /well and 6 × 105 cells /well in 6-well plates, respectively, and incubated for 24 h. Then, cells were treated with 50% EtOH extract of S. glauca (6.25, 12.5 and 25 μg/mL) or 10 μM of each compound or finasteride (positive control, 10 or 20 μM). After 1 h, cells were treated with 0.5 μM of testosterone propionate (TP). LNCaP and RWPE-1 cells were incubated for 72 h and 24 h. After washing with PBS 3 times and cell lysates were extracted with an M-PERTM buffer. The protein concentration in the harvested cells was measured using the Bradford assay. 30 μg of protein samples were separated by 10% SDS-PAGE at 100 V and then, transferred to the PVDF membrane. The membrane was blocked with blocking buffer (5% skim milk containing 150 mM NaCl, 10 mM Tris, and 0.05% tween 20 in PBS) for 1 h at room temperature, then incubated overnight with a primary antibody at 4℃. The following primary antibodies used; AR (5153T, Cell Signaling Technology), PCNA (13110S, Cell Signaling Technology), 5αR2 (sc-293232, Santa Cruz Biotechnology), PSA (5365S, Cell Signaling Technology), α-tubulin (sc-8035, Santa Cruz Biotechnology) and β-actin (sc8432, Santa Cruz Biotechnology). After washing Tris Buffered Saline with Tween 20 (TBS-T) 3 times, the membrane was incubated for 1 h at room temperature with secondary antibody conjugated mouse anti-rabbit IgG or goat anti-mouse IgG (1:10,000 dilution; Santa Cruz Biotechnology). After washing with TBS-T 3 times, bands were detected with an ECL solution (Bio-Rad clarity Max western ECL substrate) and quantified using Chemidoc Imaging System (Fusion FX5, Vilber Lourmat, France).
Statistical analysis – Data of three different experiments are expressed as mean ± SD. Statistical significance was analyzed using one-way ANOVA followed by Dunnett’s t-tests (p < 0.05). Data was analyzed using GraphPad Prism ver. 5 (San Diego, CA, USA).
Results and Discussion
Testosterone and DHT are androgens known to be related to the development of BPH. Testosterone is converted to DHT by 5αR2 and DHT exhibits more potent androgenic activity than testosterone due to its greater affinity for AR.28,29 DHT binds to AR, resulting in increased levels of PSA, which tends to rise in cases of BPH and prostate cancer.30 PSA levels are a key biomarker in the clinical evaluation of prostate cancer and have been reported to be helpful in assessing prostate volume and the risk of progression in BPH.31 Furthermore, PCNA, a critical role in cell proliferation, and is elevated in testosterone propionate (TP)-induced BPH models, suggesting its involvement in the proliferative processes related to BPH.32 In the process of exploring new natural materials from halophytes for the prevention or treatment of BPH, the extract of S. glauca was found to exhibit excellent effects, leading to subsequent phytochemical and biological studies on this plant.
S. glauca is classified as a halophyte in the family of Amaranthaceae and is known to be distributed along the coasts of Asia. The isolation of flavonoids, pentacyclic triterpenoids, steroids, phenolics, and coumarins has been reported in previous phytochemical studies on S. glauca.19–22 The various physiological activities of S. glauca extract and its derived constituents have been reported, with research predominantly focusing on its antioxidant, antiinflammatory, and hepatoprotective effects. Han et al.,24 demonstrated that the extracts of S. glauca cultivated in a smart farm showed anti-fibrotic effects through the inhibition of TGFβ1-Smad2/3 signaling in hepatic stellate cells and in CCl4-induced C57BL/6 mice. It was reported that ethanol extracts and solvent fractions of S. glauca leaves exhibited antioxidant effects, and that the chloroform fraction significantly suppressed the expression of markers related to inflammation in LPS-induced RAW cells, thereby inhibiting NO production.25 Among the compounds derived from S. glauca, potent antioxidant activity of suaeglaucin B20 and the hepatoprotective effect of methyl 3,5-di-O-caffeoyl quinate in HepG2 cells22 have been described.
In this study, eight compounds; N-trans-feruloyltyramine (1), N-feruloyl normetanephrine (2), trolline (3), kaempferol (4), kaempferol-3-O-β-D-glucoside (5), quercetin-3-O-β-D-glucoside (6), tyrosol (7) and hydroxytyrosol (8) isolated and identified from S. glauca were used to determine the major components in the HPLC chromatogram of ESG. Additionally, the effects of ESG and the compounds 1–8 on improving BPH were evaluated using TP-activated RWPE-1 and LNCaP cells.
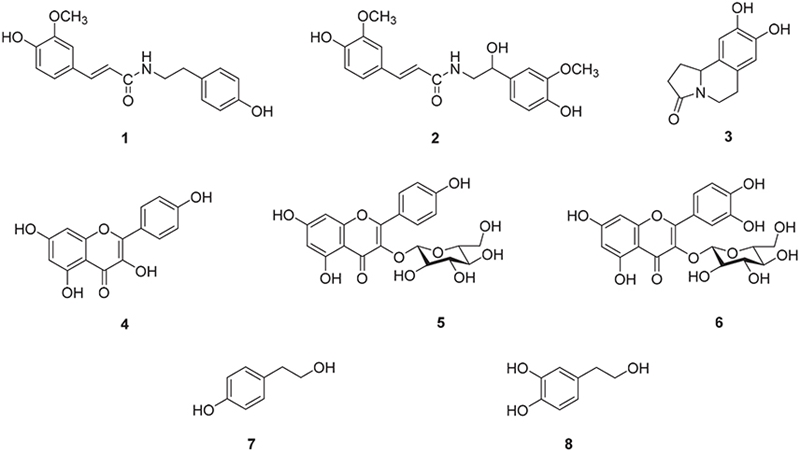
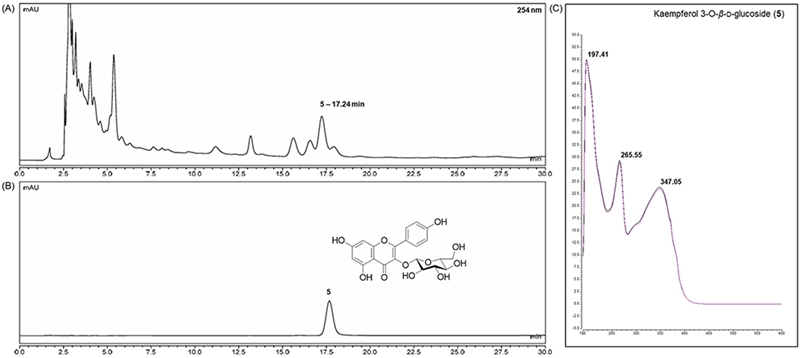
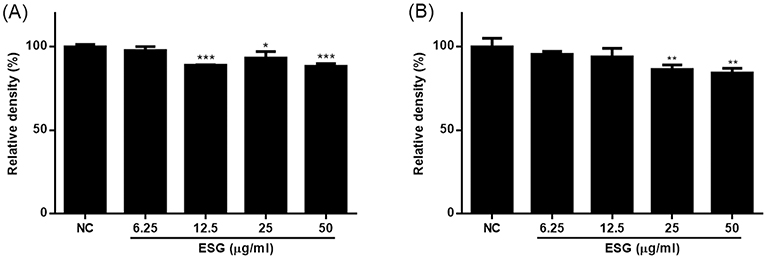
Chemical profile of ESG was identified by comparing the retention time and UV spectrum of the compounds 1–8 using HPLC-DAD. As shown in Fig. 2, five major peaks were observed in the HPLC chromatogram of ESG and one of them was identified as kaempferol-3-O-β-D-glucoside (5) which was detected at 254 nm with RT of 17.24 min. The UV spectra and RT of the remaining compounds were not found to match exactly with the peaks detected in the spectrum of ESG. The effects of ESG and the isolated compounds in treating or ameliorating BPH were evaluated through the regulation on the expressions of BPH-related biomarkers in LNCaP and RWPE-1 cells. Prior to evaluating the effects of ESG, the cytotoxicity of ESG was measured using MTT assay. As shown in Fig. 3, the treatment of cells with ESG showed no significant toxicity at concentration range of 6.25–25 μg/mL against RWPE-1 and LNCaP cells. Based on MTT assay, the effects of ESG on the expressions of AR, PCNA and 5αR2 were evaluated in TP-activated RWPE-1 cells, and on the expressions of AR and PSA in TP-activated in LNCaP cells. In RWPE-1 cells, treatment with the ESG was found to reduce the expressions of AR, 5αR2, and PCNA, with significant changes observed at a high concentration (25 μg/mL). Whereas in LNCaP cells, the reduction in AR and PSA expressions was observed from the concentration of 6.25 μg/mL, with effects comparable to or superior to those of the positive control, finasteride (Fig. 4 and 5). Based on the effects of ESG to suppress the expressions of AR, PCNA, PSA and 5αR2, the potentials of compounds 1–8 isolated from S. glauca were further evaluated. As shown in Fig. 6, no significant toxicity was observed in both cells by compounds at the concentration range from 0.1 to 10 μM (the result of 10 μM was only shown) in which the cell viability was over 90%. In RWPE-1 cells, AR expression was inhibited by 1 and 8, PCNA expression was suppressed by 2–4, and 5αR2 expression was inhibited by 3 and 8. Notably, the inhibition of AR by 8 was most remarkable with protein expression reduced to levels below those of NC group (Fig. 7). In LNCaP cells, all compounds except 6 were observed to exhibit significant inhibitory effects on AR or 5αR2. For PSA expression, 1, 2, and 8 demonstrated strong inhibitory effects, with 8 showing the most pronounced activity (Fig. 8). Based on these results, 8 is inferred to exhibit the most potent activity on BPH-related biomarkers among the eight compounds isolated from S. glauca in the two prostate cell lines, RWPE-1 and LNCaP. Hydroxytyrosol, a phenolic compound sourced from the olive tree and its leaves, is commonly obtained as a by-product during olive oil production. It is regarded as one of the most powerful antioxidants among olive-derived phenolic compounds and is linked to various health benefits, such as anti-oxidant, anti-inflammatory, anti-cancer effects, and protection for the skin and eyes.33–36 In relation to the prostate, hydroxytyrosol has demonstrated antiproliferative effects on prostate cancer cell lines such as PC-3, LNCaP, and 22Rv1 cells.37–39 However, its activity against BPH has not been previously reported. In this study, we observed that hydroxytyrosol (8) effectively reduced the expression of BPH-related biomarkers in both normal prostate epithelial cells and prostate cancer cells. These findings suggest the need for further research to clarify the underlying mechanisms and assess its clinical significance.
HPLC chromatogram of the 50% ethanol extract of S. glauca (A) and kaempferol-3-O-β-D-glucoside (5) (B), and UV-Vis spectrum of 5 (C).
The cytotoxicity of 50% EtOH extract of S. glauca (ESG) in RWPE-1 (A) and LNCaP (B) cells. The cell viability was measured by MTT assay. Cells were incubated with ESG at the concentrations of 6.25, 12.5 and 25 μg/mL for 24 h. Results are presented as the mean ± S.D. of triplicate experiments.
The effects of 50% EtOH extract of S. glauca (ESG) on the expressions of AR (A), PCNA (B) and 5αR2 (C) in RWPE-1 cells activated with TP. Cells were treated with TP (0.5 μM) and then treated with 50% EtOH extract of S. glauca (6.25, 12.5 and 25 μg/mL) or finasteride (20 μM) for 24 h. The expression levels of AR, PCNA and 5αR2 in cells were analyzed by western blot. Results are presented as the mean ± S.D. of triplicate experiments; *p < 0.05, **p < 0.01 and ***p < 0.001 compared to TP-treated cells. AR: androgen receptor, PCNA: proliferating cell nuclear antigen, 5αR2: 5-alpha reductase type 2, TP: testosterone propionate, Fina: finasteride, NC: non-treated control.
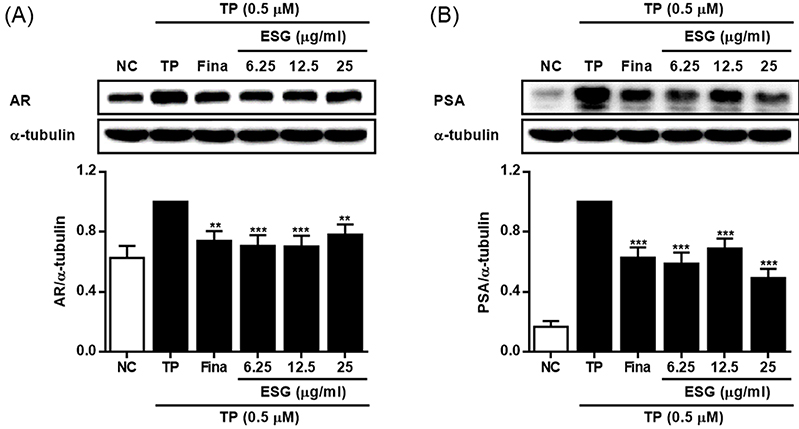
The effects of 50% EtOH extract of S. glauca (ESG) on the expressions of AR (A) and PSA (B) in LNCaP cells activated with TP. Cells were treated with TP (0.5 μM) and then treated with 50% EtOH extract of S. glauca (6.25, 12.5 and 25 μg/mL) or finasteride (10 μM) for 72 h. The expression levels of AR and PSA in cells were analyzed by western blot. Results are presented as the mean ± S.D. of triplicate experiments; **p < 0.01 and ***p < 0.001 compared to TP-treated cells. AR: androgen receptor, PSA: prostate specific antigen, TP: testosterone propionate, Fina: finasteride, NC: non-treated control.
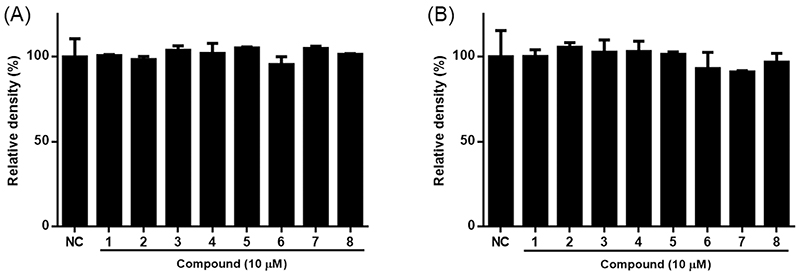
The cytotoxicity of 1–8 isolated from S. glauca in RWPE-1 (A) and LNCaP (B) cells. The cell viability was measured by MTT assay. Cells were treated with each compound (10 μM) for 24. Results are presented as the mean ± S.D. of triplicate experiments.
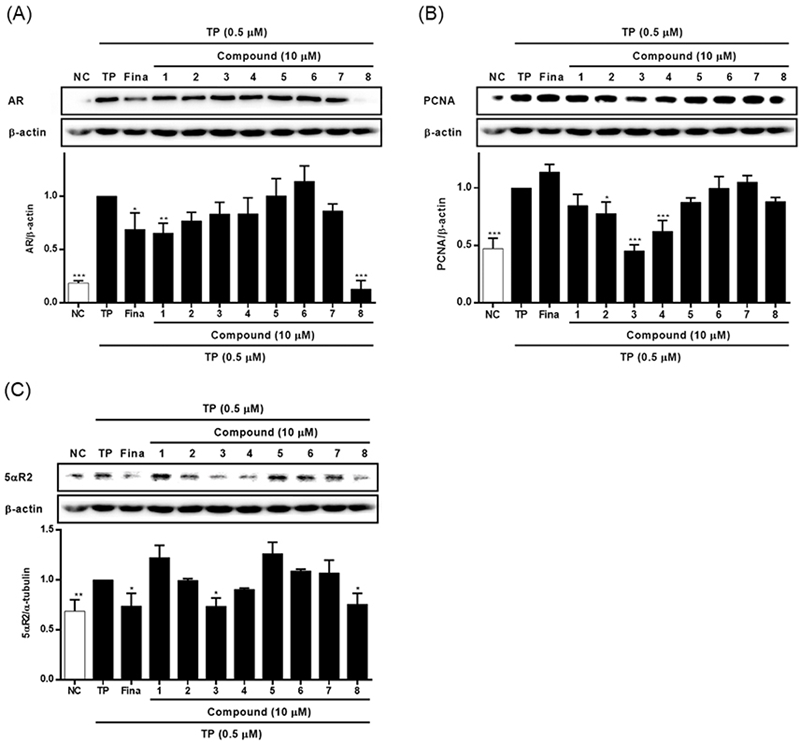
The effects of 1–8 isolated from S. glauca on the expressions of AR (A), PCNA (B) and 5αR2 (C) in RWPE-1 cells activated with TP. Cells were treated with TP (0.5 μM) and then treated with each compound (10 μM) or finasteride (20 μM) for 24 h. The expression levels of AR, PCNA and 5αR2 in cells were analyzed by western blot. Results are presented as the mean ± S.D. of triplicate experiments; *p < 0.05, **p < 0.01 and ***p < 0.001 compared to TP-treated cells. AR: androgen receptor, PCNA: proliferating cell nuclear antigen, 5αR2: 5-alpha reductase type 2, TP: testosterone propionate, Fina: finasteride, NC: non-treated control.
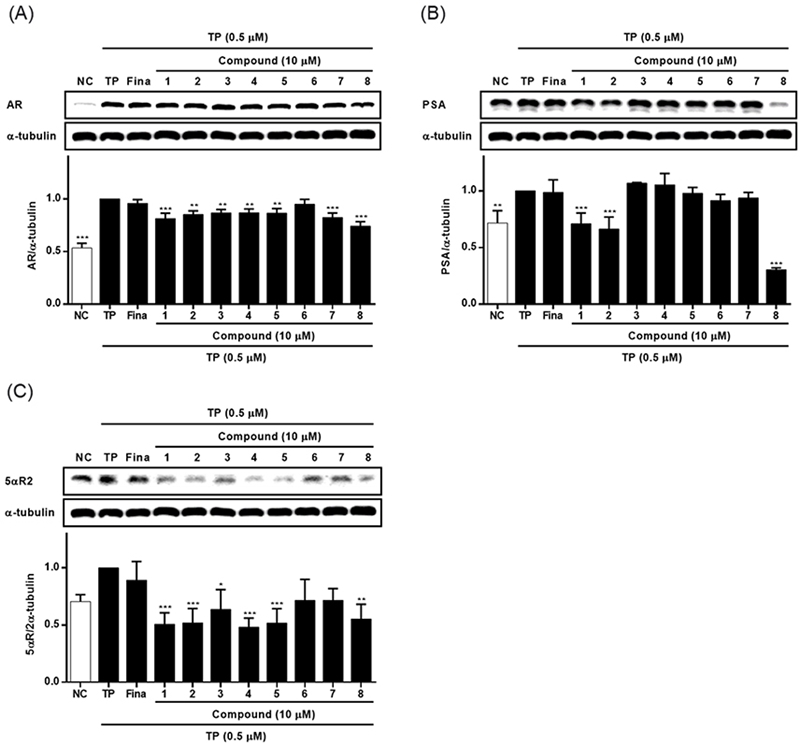
The effects of compounds 1–8 isolated from S. glauca on the expressions of AR (A), PSA (B) and 5αR2 (C) in LNCaP cells activated with TP. Cells were treated with TP (0.5 μM) and then treated with each compound (10 μM) or finasteride (10 μM) for 72 h. The expression levels of AR, PSA and 5αR2 in cells were analyzed by western blot. Results are presented as the mean ± S.D. of triplicate experiments; *p < 0.05, **p < 0.01 and ***p < 0.001 compared to TP-treated cells. AR: androgen receptor, PSA: prostate specific antigen, 5αR2: 5-alpha reductase type 2, TP: testosterone propionate, Fina: finasteride, NC: non-treated control.
In conclusion, ESG and its derived compounds exhibited effective potential in improving or treating BPH, with particularly notable effects observed for hydroxytyrosol.
Acknowledgments
This research was a grant from the National Research Foundation of Korea (NRF) NRF-2023R1A2C1007448 and by a grant from the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Ocean and Fisheries KIMST-20220526.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
-
Madersbacher, S.; Sampson, N.; Culig, Z. Gerontology 2019, 65, 458–464.
[https://doi.org/10.1159/000496289]

-
Marszalek, M.; Ponholzer, A.; Pusman, M.; Berger, I.; Madersbacher, S. Eur. Urol. Suppl. 2009, 8, 504–512.
[https://doi.org/10.1016/j.eursup.2009.02.003]

-
Mobley, D.; Feibus, A.; Baum, N. Postgrad. Med. 2015, 127, 301–307.
[https://doi.org/10.1080/00325481.2015.1018799]

-
Pushkar, D. Y.; Rasner, P. I. Urologia 2017, s3, 4–18.
[https://doi.org/10.18565/urol.2017.3-supplement.4-18]

-
McVary, K. T.; Roehrborn, C. G.; Avins, A. L.; Barry, M. J.; Bruskewitz, R. C.; Donnell, R. F.; Foster, H. E.; Gonzalez, C. M.; Kaplan, S. A.; Penson, D. F.; Ulchaker, J. C.; Wei, J. J. Urol. 2011, 185, 1793–1803.
[https://doi.org/10.1016/j.juro.2011.01.074]

-
Izumi, K.; Mizokami, A.; Lin, W.-J.; Lai, K.-P.; Chang, C. Am. J. Pathol. 2013, 182, 1942–1949.
[https://doi.org/10.1016/j.ajpath.2013.02.028]

- Banerjee, P. P.; Banerjee, S.; Brown, T. R.; Zirkin, B. R. Am. J. Clin. Exp. Urol. 2018, 6, 62–77.
-
Roehrborn, C. G. Int. J. Impot. Res. 2008, 20, S11–S18.
[https://doi.org/10.1038/ijir.2008.55]

-
Kang, P. M.; Kim, Y. J.; Seo, W. T.; Kang, S. H.; Kim, T. S.; Chun, B. K.; Seo, W. I.; Jeong, J.-Y.; Chung, J. World J. Urol. 2019, 37, 709–718.
[https://doi.org/10.1007/s00345-018-2422-4]

-
Bartsch, G.; Rittmaster, R. S.; Klocker, H. Eur. Urol. 2000, 37, 367–380.
[https://doi.org/10.1159/000020181]

-
Andriole, G.; Bruchovsky, N.; Chung, L. W. K.; Matsumoto, A. M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. J. Urol. 2004, 172, 1399–1403.
[https://doi.org/10.1097/01.ju.0000139539.94828.29]

-
Carson III, C.; Rittmaster, R Urology 2003, 61, 2–7.
[https://doi.org/10.1016/S0090-4295(03)00045-1]

-
Kiguradze, T.; Temps, W. H.; Yarnold, P. R.; Cashy, J.; Brannigan, R. E.; Nardone, B.; Micali, G.; West, D. P.; Belknap, S. M. PeerJ 2017, 5, e3020.
[https://doi.org/10.7717/peerj.3020]

-
Fertig, R. M.; Gamret, A. C.; Darwin, E.; Gaudi, S. Dermatol. Online J. 2017, 23.
[https://doi.org/10.5070/D32311037240]

-
Oyama, N.; Kaneko, F. J. Am. Acad. Dermatol. 2009, 60, 168–169.
[https://doi.org/10.1016/j.jaad.2008.07.037]

-
Traish, A. M. World J. Mens Health 2020, 38, 323–337.
[https://doi.org/10.5534/wjmh.200012]

-
Yi, L.; Sa, R.; Zhao, S.; Zhang, X.; Lu, X.; Mu, Y.; Bateer, S.; Su, S.; Wang, S.; Li, Z.; Shi, S.; Zhao, X.; Lu, Z. Front. Genet. 2022, 13, 884081.
[https://doi.org/10.3389/fgene.2022.884081]

-
Qu, X.-J.; Liu, L.-K.; Zhang, L.-Y.; Zhang, X.-J.; Fan, S.-J. Mitochondrial DNA B. Resour. 2019, 4, 2780–2781.
[https://doi.org/10.1080/23802359.2019.1659111]

-
Wang, Q.-Z.; Qiu, P.; Liu, F.; Wang, B.; Guan, F.-Q.; Feng, X.; Xu, S. J. Asian Nat. Prod. Res. 2018, 20, 1081–1087.
[https://doi.org/10.1080/10286020.2017.1415330]

-
Qiu, P.; Guan, F.; Feng, X.; Liu, F.; Liu, X.; Jin, T.; Lyu, H.; Huang, J.; Tong, H.; Chen, M.; Wang, B.; Wang, Q. Chem. Nat. Compd. 2021, 57, 16–19.
[https://doi.org/10.1007/s10600-021-03270-9]

-
Wang, Q.; Qiu, P.; Guan, F.; Shan, Y.; Yin, M.; Feng, X.; Liu, F. Chem. Nat. Compd. 2018, 54, 38–40.
[https://doi.org/10.1007/s10600-018-2254-x]

-
An, R.-B.; Sohn, D.-H.; Jeong, G.-S.; Kim, Y.-C. Arch. Pharm. Res. 2008, 31, 594–597.
[https://doi.org/10.1007/s12272-001-1198-1]

- Lee, K.-S.; Park, K.-S. Korean J. Food Nutr. 2019, 32, 581–588.
-
Hong, Y.-J.; Kim, G.-H.; Park, Y.; Jo, H.-J.; Nam, M.-W.; Kim, D.-G.; Cho, H.; Shim, H.-J.; Jin, J.-S.; Rho, H.; Han, C.-Y. Nutrients 2023, 15, 3740.
[https://doi.org/10.3390/nu15173740]

- Park, J. M.; Kim, S. D.; Lee, W. M.; Cho, J. Y.; Park, H. J.; Kim, T. W.; Choe, N.-H.; Kim, S. K.; Rhee, M. H. Pharmazie 2007, 62, 453–458.
-
Lee, S.-G.; Kim, J.-B.; Kang, H. Trop. J. Pharm. Res. 2016, 15, 1175–1181.
[https://doi.org/10.4314/tjpr.v15i6.9]

-
Kim, Y.-N.; Park, M.-G.; Kim, Y.-J.; Lee, J.-S.; Kwon, B.-O.; Rho, J.-R.; Jeong, E.-J. Molecules 2024, 29, 298.
[https://doi.org/10.3390/molecules29020298]

-
Thigpen, A. E.; Silver, R. I.; Guileyardo, J. M.; Casey, M. L.; McConnell, J. D.; Russell, D. W. J. Clin. Invest. 1993, 92, 903–910.
[https://doi.org/10.1172/JCI116665]

-
Roehrborn, C. G.; Boyle, P.; Nickel, J. C.; Hoefner, K.; Andriole, G. Urology 2002, 60, 434–441.
[https://doi.org/10.1016/S0090-4295(02)01905-2]

-
Levitt, J. M.; Slawin, K. M. Curr. Urol. Rep. 2007, 8, 269–274.
[https://doi.org/10.1007/s11934-007-0072-y]

-
Tanguay, S.; Awde, M.; Brock, G.; Casey, R.; Kozak, J.; Lee, J.; Nickel, J. C.; Saad, F. Can. Urol. Assoc. J. 2009, 3, S92–S100.
[https://doi.org/10.5489/cuaj.1116]

-
Bae, J.-S.; Park, H.-S.; Park, J.-W.; Li, S.-H.; Chun, Y.-S. J. Nat. Med. 2012, 66, 476–485.
[https://doi.org/10.1007/s11418-011-0609-8]

-
Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A. F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Antioxidants 2020, 9, 781.
[https://doi.org/10.3390/antiox9090781]

-
Arangia, A.; Marino, Y.; Impellizzeri, D.; D’Amico, R.; Cuzzocrea, S.; Di Paola, R. Int. J. Mol. Sci. 2023, 24, 3111.
[https://doi.org/10.3390/ijms24043111]

-
Costantini, F.; Di Sano, C.; Barbieri, G. Int. J. Mol. Sci. 2020, 21, 8074.
[https://doi.org/10.3390/ijms21218074]

-
Martínez, L.; Ros, G.; Nieto, G. Medicines 2018, 5, 13.
[https://doi.org/10.3390/medicines5010013]

-
León-González, A. J.; Sáez-Martínez, P.; Jiménez-Vacas, J. M.; Herrero-Aguayo, V.; Montero-Hidalgo, A. J.; Gómez-Gómez, E.; Madrona, A.; Castaño, J. P.; Espartero, J. L.; Gahete, M. D.; Luque, R. M. Antioxidants 2021, 10, 1348.
[https://doi.org/10.3390/antiox10091348]

-
Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; Long, J; Liu, J. Curr. Cancer Drug Targets 2013, 13, 625–639.
[https://doi.org/10.2174/15680096113139990035]

-
Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S. K.; Khan, M. A.; Patel, G. K.; Singh, S.; Singh, A. P. Nutr. Cancer 2017, 69, 932–942.
[https://doi.org/10.1080/01635581.2017.1339818]